Recent Posts
- Peace Through Water Desalination
- CPAC Water Policy Interview with KLRN Radio San Antonio Texas
- CPAC Water Interview With California Talk Show Host Rick Trader
- Toward a Green Earth Policy in the era of Trump
- Gates Foundation Water Energy Vision
Recent Comments
- on LLNL Researchers use carbon nanotubes for molecular transport
- on Greenhouses for Desalination
- on American Membrane Technology Association
- on Engineers develop revolutionary nanotech water desalination membrane
- on LLNL Researchers use carbon nanotubes for molecular transport
Archives
- May 2017
- March 2017
- June 2011
- December 2008
- November 2008
- October 2008
- September 2008
- August 2008
- July 2008
- June 2008
- April 2008
- February 2008
- January 2008
- December 2007
- November 2007
- October 2007
- September 2007
- August 2007
- July 2007
- June 2007
- May 2007
- April 2007
- March 2007
- February 2007
- January 2007
- December 2006
- November 2006
- October 2006
- September 2006
- August 2006
- July 2006
- June 2006
Categories
Saltwater into fire
01st June 2007
You really have to see this to believe it. No that’s likely not good enough. You’ll have to get your own low energy radio wave machine and toast some salt water yourself. That’s what’s John Kanzius has done. The Sanibel Island Florida inventor was looking for a way to desalinate water but instead found a way to burn salt water. According to reports low energy radio waves split the H20 up into hydrogen & oxygen. It looks like The Na & Cl in solution acted as a heat sink or electrolyte . Or anyhow I assume so since the process wouldn’t work on fresh water. Maybe the RF acts as the catalyst as this discussion suggests. See below for the answer. One way or another -something bubbled out of solution. And with a flick of a bic the gases turned to fire.
Anyhow here’s a couple videos.
Salt Water into Fuel
Saltwater into fire 2
Saltwater into fire 3
Saltwater into fire 4
Understand. Electrolysis results in a net loss of energy. ie you put in more energy than you get out. Do not conflate electrolysis with radio waves. Radio waves represent a much smaller energy input.
Small enough to make for a net gain in energy?
For anyone with serious math skills, it should be possible to do a rough–back of the envelope– calculation based on the comments of this article:
Charles Rutkowski placed a test tube filled with ordinary salt water into John Kanzius’ external radio-wave generator.
He then blasted the salt water with 200 watts’ worth of directed radio waves, not quite enough electricity to light three 75-watt light bulbs.
Within seconds, a blue flame erupted from the top of the test tube. It then turned bright white like a blowtorch’s flame and burned for several minutes at about 3,000 degrees Fahrenheit.
“I’ve done this countless times and it still amazes me,” said Rutkowski, general manager of Industrial Sales and Manufacturing, the Millcreek company that builds Kanzius’ generators.
So what’s going on?
For starters, here’s a simple experiment that shows that salt increases many fold the output of hydrogen from electrolysis. (The Kansius experiment does not involve electrolysis but rather Radio Waves).
Here is a second bit of fun with a microwave that shows that the microwave can turn a flame off and on at will and make the flame burn high & hot. You can see in Kansius’s salt water burning video how Kansius turns the high hot fire off and on at will. The suggestion here is that the radio waves are both splitting the water and then further exciting the flames. As well, I’m suggesting that the process is analogous to that in a microwave oven.
Update:I talked on the phone to one of the scientists Ed Apsega at APV Engineering in Akron Oh. They tested John Kanzius process. I was told the flame burned at more than 1700 degrees Celcius or 3000 degrees Farenheit. (Its not clear to me currently as to whether the energy yield is more or less than 1:1. Why? Well the APV engineering scientist Ed Apsega said the energy yield was much more than 2:1 and later I talked to John Kanzius–he said the energy yield was less than 1:1.) The yellow flame was the glass burning. (The flame started out clear.) The temperature inches away from the flame was room temperature. Tests afterwards showed that the water was reduced and the mineral content by percent increased in the water. The Na in the water decreased but not significantly. John Kanzius would like government money for his salt water project so that he can work on his cure for cancer. I’ve been promised a follow up email/phone call from Kanzius so that he can provide a contact number/email that I can post. So check back.Update: Ok I talked with John Kanzius. He’s ok with being reached at johnkanzius (at) aol.com. There are three machines available. It would be helpful if someone qualified checked this thing out.
According to this site:
June 01, 2007
“Regarding moving this forward, I want to see what are the best results we can achieve with joules in vs joules out. A chemist in Houston whom I know is going to be doing a couple of things for me this weekend.” — John Kanzius (June 01, 2007)
“What burns at a temperature of over 1700 C? [Knowing the answer to that question] might take some of the guess work out of the equation.” (May 29, 2007)
June 06, 2007
John Kanzius writes:
“Since it appears we now have now achieved more than unity, I am going to do an embargo on releasing all further information.
“Actually there are smart individuals who have posted on different web sited and actually have a pretty good idea of what is happening.”
……………….
So why is the flame so high? Why doesn’t the wick burn? Why does the temperature so near to the flame revert back to room temperature? (See below.) The answer to all three questions seems to be that the flame shown in the video is an electrical fire. Some are calling it a plasma fire.
Slide Three of this slide show has a still of the Therm Med LLC External RF System. The machine is proprietary radio frequency machine. You’ll have to check with John Kanzius about that. See above.It appears that the secret sauce in the process is in the RF frequency. The frequency itself is the catalyst.
Consider this patent on the process: (It looks like somehow the radio waves immitate platinum.)
Catalytic simulation using radio frequency waves
Document Type and Number:
United States Patent 6217712
Link to this page:
http://www.freepatentsonline.com/6217712.html
Abstract:
The invention relates to a method of using radio frequency waves to artificially create catalytic action in a catalyst-free chemical reaction within a substance. To mimic or imitate the catalyst, radio frequency waves are transmitted through the substance at a signal strength sufficient to electronically reproduce the effect of the physical presence of a selected catalyst. The radio frequency waves have a selected transmission frequency substantially equal to a catalyst signal frequency of the selected catalyst, defined as the signal frequency determined by nuclear magnetic resonance of the selected catalyst. It is commonplace to use nuclear magnetic resonance to identify elements within a substance and the signal frequencies of various elements (including catalysts) are listed in widely published tables. To date, the mechanism by which catalysts bring about chemical reactions has been unknown. The inventor has recognised that the physical presence of a catalyst brings about a chemical reaction due to the emission of low intensity radio frequency waves from the catalyst with the signal frequency that is emitted being the signal frequency of the catalyst that is commonly determined by nuclear magnetic resonance. Therefore, the invention can be used to eliminate the need for expensive metallic catalysts, such as platinum. The invention electronically reproduces the effect of the physical presence of a catalyst by transmission of a radio frequency wave with a signal frequency equal to that signal frequency emitted by the catalyst and as determined by nuclear magnetic resonance of the catalyst.
Here is a list of his patents related to the process.
more on patents:
download PDF (Systems and methods for combined RF-induced hyperthermia and radioimmunotherapy, 2005)
download PDF (Enhanced systems and methods for RF-induced hyperthermia II, 2006)
European Patent Office (Kanzius patents)
WIPO.int (Kanzius patents)
European Patent Office RASBACH KLAUS (DE) This is an expired patent. I believe this gives the key.
In resonance-based generation of H2 and O2 from water, using a hypersonic generator of suitable frequency, the resonance frequency (fO) can be that corresp. to the distance (d) between the nucleus of the O atoms and its outer electron shell or the proton. fO can be calculated approx. from the formula: fO = c/(pi.d), where c= the speed of sound in water and pi= the Ludolf’s no). USE/ADVANTAGE – The H2 can be used as fuel in power stations or in hydrogenation (hardening fat), synthesis of petrol, MeOH and NH3, redn in metallurgy, in welding etc. The O2 can also be used for technical and other purposes. The overall efficiency of the process is much higher than usual and the process is more friendly to the environment.
According to this poster:
“Perhaps if we could find a substance with a NMR frequency of 13.56 mhz then that is our catalyst…”. Now if you can just turn that around a bit–you might get that the frequency that the machine is imitating is that produced by platinum or 13.56 mhz.
According to this poster:
From what I understand of this, (1) day reading, is that every metal has a moleculer frequency. The patent says that these numbers are readily available.
Next, you take your RF signal generator and set it to that frequency and put it into the solution according to the patent.
The solution “feels” the catalyst. If it had a brain it would be tricked into thinking it is seeing that particular metal.
My first thought is KOH in water and Aluminum. Replace the Aluminum with the molecular resonant frequency of the Aluminum metal and you have the KOH water mixture thinking you just put in AL. And here comes Hydrogen!! Can you say,”unbelievable!” Where has this tech been?!
Here are additional links:
Fla. Man Invents Machine To Turn Water Into Fire
How John Kanzius’ push to cure cancer may have discovered alternate fuel
Here’s a google search for Kanzius+burn+water.
“On our way to try to do desalinization, we came up with something that burns, and it looks in this case that salt water perhaps could be used as a fuel to replace the carbon footsteps that we’ve been using all these years, i.e., fossil fuels,” Kanzius said.
If it’s for real, the possible ramifications of the discovery are almost mind-boggling, as cars could be fueled by salt water instead of gasoline, hydroelectric plants could be built along the shore, and homes could be heated without worrying about supplies of oil.
“It doesn’t have to be ocean salt water,” Kanzius said. “It burns just as well when we add salt to tap water.”
Thus far, Kanzius’ work has not received extensive national publicity, but has been featured on several local television news programs, including WPBF-TV in West Palm Beach, Fla., WSEE-TV in Erie, Pa., and WKYC-TV in Cleveland. “We discovered that if you use a piece of paper towel as a wick, it lights every single time and you can start it and stop it at will by turning the radio waves on and off,” Kanzius told the Times-News as he watched a test tube of salt water burn.
“And look, the paper itself doesn’t burn,” he added. “Well, it burns but the paper is not consumed.”
Kanzius said he hasn’t decided whether to share his fuel discovery with government or private business, though he’d prefer a federal grant to develop it.
uh…. can someone fit this man’s work into their budget? I’m sure this could be worked around for water desalination/transport purposes.
Another but dissimilar process was announced recently where an aluminum-gallium alloy acted as the catalyst: (ie no RF was needed to make the reaction. But you might be able to tune radio wave to immitate the RF of aluminum-gallium)
I have blogged about working on desalination catalysts before but not really from the angle approached here. You can do a quick search on google for RF +deposition OR RF “water splitting” OR RF semiconductor. It looks like the semiconductor industry uses RF energy to split gas molecules, then recombines them to deposit chemical films onto wafers. According to this poster
I’ve seen another piece of equipment which uses magnetic
fields to direct ionized gases. I imagine this might be one method to
harvest the hydrogen before it has a chance to recombine with oxygen.
You might also tune RF to settle Na & Cl ions out of solution. Just a thought.
Last month the New York Times published an article about how the West is likely entering a prolonged period of water shortages. Similiar reports have recently been published in Australia detailing expected extended droughts over the next 50 years.
The USA and Australia have responded to these reports in different ways.
Several weeks ago I blogged about the current administration’s effort to push bulk water tranfers from Canada. This week Australia announced they were about to embark on a major desalination research project with the view of spending $250 million over seven years and cutting energy costs for desalination in half. Interestingly, their belief that such results are doable is based on recent research in the US. Further, they considered long distance bulk water transfers. They concluded, however, that doing the research to lower the cost of desalination was less expensive and complicated. And too, the ocean is a more reliable resource. See my concluding remarks after the article posted below.
Thirsty Australia Advances Desalination Technology
MELBOURNE, Australia, May 18, 2007 (ENS) – The delivery of energy efficient water desalination to drought-stricken Australia received a boost today with the establishment of a new collaboration between the government research agency CSIRO and nine Australian universities.
The research aims to advance water desalination as an alternative water supply option for Australia by increasing efficiency, and reducing the financial and environmental costs of producing desalinated water.
Australia, especially southern Australia, is short of water, and the country is experiencing the worst drought on record this year. Desalination of seawater is a possible additional supply, but it requires a lot of electricity, and is expensive, costing about A$1.10 per 1,000 liters (US$.90 per 264 gallons).
The new research effort, known as the Advanced Membrane Technologies for Water Treatment Research Cluster, is led by Professor Stephen Gray of Victoria University.
As a first step, the multi-disciplinary research team will carry out an evaluation of existing membranes and develop new energy efficient membranes.

Professor Stephen Gray is director of the Institute of Sustainability and Innovation at Victoria University, where he is responsible for research, education and industry liaison in the water, energy and sustainable buildings sectors. (Photo courtesy ISI)
“Many desalination and recycling programs rely on a process called reverse osmosis, where the water is forced through a semi-permeable membrane, removing salts and any other contaminants,” Gray explains.”These membranes need regular replacement and cleaning, but they also require a large amount of energy to force water through what are nano-sized pores,” he says.
When contaminants such as salts are removed from water, some of them adhere to the surface of the membrane, building up on the surface, increasing the pressure and energy required to desalinate the water.
“Chemicals are used to clean the membranes, but membrane surfaces that are less sticky would reduce the pressure and energy required and the frequency of cleaning,” Gray says.
The researchers aim to improve membrane anti-fouling properties, increasing the ability of the membranes to clean themselves without chemicals.
The research will link with and inform related CSIRO research into membrane and carbon nanotube water filtration technologies.
Carbon nanotubes, molecules made of carbon atoms, are hollow and more than 50,000 times thinner than a human hair. Billions of these tubes serve as the pores in a desalination membrane.

Carbon nanotubes can be made in many different configurations. (Photo courtesy Softpedia)
The smooth inner walls of the nanotubes allow liquids and gases to rapidly flow through, while the miniscule pore size keeps out larger molecules.Alan Gregory, urban water research leader at CSIRO, says, “In combination with other research projects led by CSIRO, we aim to reduce by up to 50 percent the amount of energy required to desalinate seawater using membranes. This same technology will have benefits for the treatment and recycling of wastewater.”
CSIRO researchers are using nanotechnology to develop a new membranes for desalination with electrodialysis technology, which they say may lead to breakthrough technologies in cost-effective and highly efficient water recovery systems.
Nanotechnology for water desalination is a rapidly developing field. In the United States, researchers at Lawrence Livermore National Laboratory announced in May 2006 their creation of a membrane made of carbon nanotubes and silicon that may offer less expensive desalinization.
The CSIRO scientists are developing new “inorganic-organic nanocomposite membranes for desalination by electrodialysis membrane process, which involves the incorporation of oxide nanoparticles into ion-conducting polymers to form new nanocomposites.”
“This also means we could potentially provide more secure water supplies while minimizing greenhouse gas emissions,” said Gregory.
Other partners in the membrane research program are the University of New South Wales, Monash University, the University of Melbourne, RMIT University, Curtin University of Technology, the University of Queensland, Deakin University, and Murdoch University.
Funding for the research was announced by Minister for Education, Science and Training Julie Bishop under the Flagship Collaboration Fund.
Desalination membrane advances cannot come soon enough for Australia, which is opening giant desalination plants already based on existing membrane technology, even if the water they produce is costly.

The new Perth Seawater Desalination Plant, shown here under construction, is the largest desalination plant in the southern hemisphere. (Photo courtesy ABB)
In April, the Water Corporation of Western Australia opened the 45 gigaliter Perth Seawater Desalination Plant. The US$290 million project will guarantee 17 percent of Western Australia’s current water needs, regardless of rainfall or drought.On Tuesday Western Australia Premier Alan Carpenter announced that a second desalination plant of the same size would be built at Binningup.
Meanwhile, the New South Wales Government of Premier Morris Iemma is moving forward with a huge desalination plant south of Sydney at Kurnell. The plant will use reverse osmosis technology with membranes that remove salts and other impurities from seawater to produce drinking water.
The environmental assessment for the construction and operation of a pipeline for Sydney’s desalination plant is open for public comment to Monday May 28.
As part of the desalination project, an 18 kilometer pipeline will be constructed from Kurnell, across Botany Bay, to Erskineville.
Sydney Water Managing Director Kerry Schott said the Kurnell plant would be 100 percent powered by green energy and would guarantee Sydney’s water supply.
“Given the uncertainty of climate change and Sydney’s growing population, alternative sources of water need to be developed,” said Schott.
“The desalination plant will supply about seven percent of Sydney’s water supply by 2009 but it can be scaled up further if required,” he said. “This gives us a supply of water that does not depend on rainfall.”
…………………………………………………………………………………….
I blogged last December about the LLNL scientists visit to Australia. Every provincial newspaper in Australia had a write up on that visit. This contrasts sharply with the notice that was given to the work of the LLNL scientists in the USA. Their write ups were mostly confined to science journals. Perhaps that’s why the Bush administration is actively considering bulk water transfers rather than accelerating the pace of desalination research. The US political class simply hasn’t been told whats going on in the US labs. Its not that the info isn’t available. Unlike a year ago,the implications of current research has pushed into corporate America. Two weeks ago I posted that IBM was entering into membrane research in the belief that great strides would be made in the next five years.
More to the point, as in the USA –the Australians considered pumping water over great distances and mountains and concluded that water desalination research was the better alternative. Consider this article.
Saltwater offers best hope, says scientist
Desalination and an inland pipeline are two of the options being considered by the State Government as it grapples with Melbourne’s water shortage.
Pumping water over the Great Dividing Range would probably be as energy intensive as desalination, he said, but the supply would be less reliable.
“We believe we can significantly reduce the amount of energy needed for desalination and this will make it even more competitive,” he said.
Its a shame sober men can’t come to the same conclusions in the USA.
Carbon nanotubes to the rescue of Moore’s law
18th May 2007
One big problem currently with using carbon nanotubes is producing them in high volumes at low prices. Luckily carbon nanotubes can be used for an infinite variety of purposes. (imho carbon nanotubes will wind up being what steel was to the 19th century and plastics was to the 20th century.) Because of this great diversity of applications a lot of different industries are attacking the problem of producing carbon nanotubes in high volume at low prices. For this reason desalination membrane researchers and administrators need to look at how other research disciplines and industries are grappling with producing carbon nanotubes cheaply in volume–with an eye out to adapting their processes to making carbon nanotube desalination membranes.
Below is an article about work in Germany being done to make computer chips in volume at cost from carbon nanotubes. imho it makes for interesting reading.
May 16, 2007
Nanowerk LLC
Carbon nanotubes to the rescue of Moore’s law
(Nanowerk Spotlight) Over the next few years, semiconductor fabrication will move from the current state-of-the-art generation of 90 nanometer processes to the next 65 nm and 45 nm generations. Intel is even already working on 32 nm processor technology, code-named “Westmere”, that is expected to hit the market sometime around 2009. The result of these efforts will be billion-transistor processors where a billion or more transistor-based circuits are integrated into a single chip. One of the increasingly difficult problems that chip designers are facing is that the high density of components packed on a chip makes interconnections increasingly difficult. In order to be able to continue the trend predicted by Moore’s law, at least for a few more years, researchers are now turning to alternative materials for transistors and interconnect and one of the prime candidates for this job are single-walled carbon nanotubes (SWCNT). However, one of the biggest limitations of conventional carbon nanotube device fabrication techniques today is the inability to scale up the processes to fabricate a large number of devices on a single chip. Researchers in Germany have now demonstrated the directed and precise assembly of single-nanotube devices with an integration density of several million devices per square centimeter, using a novel aspect of nanotube dielectrophoresis. This development is a big step towards commercial realization of CNT-based electronic devices and their integration into the existing silicon-based processor technologies.
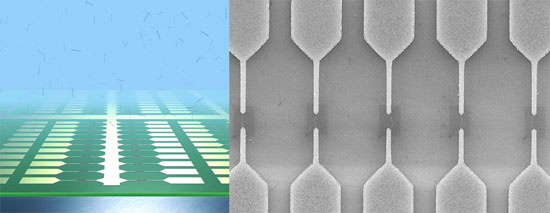
The image on the above shows a schematic of an ultra-large scale array of single-walled carbon nanotube devices fabricated by dielectrophoretic deposition from an aqueous solution. The scanning electron micrograph on the right shows the zoom-in to one region of the array showing each electrode pair bridged by an individual carbon nanotube in a self-limiting mechanism. (Images: Dr. Vijayaraghavan, Dr. Krupke, Forschungszentrum Karlsruhe)
“The fundamental issue of CNT device fabrication remains the biggest challenge for effective commercialization of nanotube electronics” Dr. Ralph Krupke explains to Nanowerk. “For CNT electronics to become a reality, it should be possible to scale up the fabrication technique to simultaneously and reproducibly fabricate a very large number of such devices on a single chip, each accessible individually for electronic transport. Conventional nanotube growth and device fabrication techniques using chemical vapor deposition or spin-casting are unable to achieve this, due to a lack of precise control over nanotube positioning and orientation.”
“Since these nanotubes are usually grown at temperatures greater then 500°C and show no growth selectivity between metallic and semi-conducting types, they can not be directly integrated into silicon-based micro-fabrication” adds Dr. Aravind Vijayaraghavan. “Due to the difficulties in handling and manipulating these nano-scale objects at the individual level, various attempts to assemble them into functional devices have met with limited success. In the ideal case, it should be possible to position an individual nanotube at a predefined location and orientation, forming robust, low-resistance, ohmic contacts to two metallic leads. Furthermore, it should be possible to do this at a scalable integration density with each nanotube forming an individually addressable device.”
Krupke and Vijayaraghavan are scientists at the Institute of Nanotechnology (INT) at the Research Center Karlsruhe in Germany. Together with colleagues from the INT and the University of Karlsruhe they authored a recent paper in Nano Letters, titled “Ultra-Large-Scale Directed Assembly of Single-Walled Carbon Nanotube Devices”. In it, they report a novel aspect of dielectrophoretic deposition of CNTs, where the dielectrophoretic force field changes upon nanotube deposition and thereby self limits the directed assembly to a single nanotube or nanotube bundle at predefined locations.
In 2003, the group demonstrated that it is possible to deposit CNT bundles from an aqueous solution using a process called dielectrophoresis which uses inhomogeneous alternating electric fields to move and assemble nano-scale objects.
“Since then, we have made tremendous advances in understanding the dynamics of a carbon nanotube moving in such an electric field” says Vijayaraghavan. “The required inhomogeneous electric fields are generated by two opposing needle-shaped electrodes with a microscopic gap between their tips. We have discovered the mechanism that allows for a self-limiting deposition of CNTs to one per electrode pair. This happens because the first CNT that is deposited in the gap changes the electric field distribution around it incisively, leading to a repulsion of subsequent CNTs that attempt to enter the region of the gap.”
The researchers in Karlsruhe have also developed and optimized the use of capacitively coupled electrodes, which enables them to reduce their dimensions and increase the density of electrode pairs that can be incorporated on a chip.
“Together, this allows us to fabricate separately addressable, individual SWCNT devices at an integration density comparable to ultra-large scale integration” says Krupke. “This is three to four orders of magnitude greater than what has been possible so far with any other technique.”
This technique is very versatile. It is compatible with SWCNTs from any source, which are suitably dispersed in an aqueous surfactant solution. SWCNTs separated based on their length, diameter or even chirality can be readily assembled into large-scale functional arrays using this technique. The process is fully compatible with post-processing techniques and current microelectronics fabrication technologies, requires no high-temperature steps or chemical modification of the substrate or the CNT and is a one-step process that can be performed under ambient conditions.
This achievement takes CNT electronic devices a big step closer to integrating with microelectronics and expanding their scope for commercial viability. On a laboratory scale, it now allows for the fabrication of a large number of devices with identical CNT source and deposition conditions, to perform truly statistical measurements of CNT properties like electronic transport or Raman mapping.
By Michael Berger, Copyright 2007 Nanowerk LLC
Watch amazing footage of how nanotubes form
11th May 2007
Here’s an interesting piece which shows on video how carbon nanotubes form. It looks to be a follow up of some work done in March at Cambridge.
Watch amazing footage of how nanotubes form

Environmental transmission electron microscopy image sequence of carbon nanofibre growth. Drawings (lower row) indicate schematically the Ni catalyst deformation and C-Ni interface. Credit: University of Cambridge
A team of scientists led by the Department’s Dr Stephan Hofmann have successfully produced live video footage that shows how carbon nanotubes, more than 10,000 times smaller in diameter than a human hair, form.
The video sequences can be viewed by clicking the links below. They show nanofibres and nanotubes nucleating around miniscule particles of nickel and are already offering greater insight into how these microscopic structures self-assemble.
These two videos show how the nickel reacts a process called catalytic chemical vapour deposition (CVD). This is one of several methods of producing nanotubes, and involves the application of a gas containing carbon (in this case acetylene) to minute crystalline droplets referred to as “catalyst islands” (the nickel).
In conditions appropriate to creating nano-fibres, the catalyst was squeezed upwards gradually as carbon formed around it. When the application of gas was reduced to create single-walled nanotubes, the carbon instead lifted off the catalyst to form a tubular structure.
Nanotube movie 1
Nanotube movie 2
In particular, the team discovered that the carbon network is guided into tubular shape by a drastic restructuring of the nickel – the catalyst in the process. They were also able to track and time the deposition of the carbon around the nickel.
Carbon nanotubes are new building blocks enabling engineers to improve and further miniaturise everyday electronic devices like computers or mobile phones. At the moment scientists can grow nanotubes but cannot accurately control their structure.
Being able to do so is vital as it is the very structure of a nanotube that dictates its properties. The nano-scale video observations mean that scientists will be able to better understand the nucleation of nanotubes and are therefore an important step on the route towards application.
The two sequences show action taking place in real time on an astonishingly small scale. The difference in size between a single-walled nanotube and a human hair is close to the difference between the same human hair and the Eiffel Tower. The microscopic scale involved has, in the past, made it difficult to understand the growth process.
The team used X-rays produced at a synchrotron (a type of particle accelerator) and a modified high-resolution transmission electron microscope to observe and film the catalytic chemical vapour deposition process.
As the gas is applied carbon sticks to the catalyst islands forming layers of graphite. In conditions appropriate to creating nanofibres, the nickel particle was pushed upwards in a series of peristaltic movements as the carbon continued to deposit on its sides. At several points the nickel formed a cap which almost “popped” out of the forming tube, leaving a layer of graphite behind it. This process is called “bambooing”, because the resultant carbon nanofibre is a cylinder containing several cavities, each one separated by one of these graphite layers, similar in form to bamboo. Throughout the whole process, the nickel remained crystalline rather than liquid.
The team then looked at conditions more appropriate to producing single-walled carbon nanotubes, which involved less acetylene. The catalyst is not squeezed upwards. Instead, a cap of carbon formed on the top of the nickel, and gradually extended from it to form a tubular structure. The catalyst island was squeezed and reshaped by this process and was moulded by the carbon forming around it rather than retaining its original form.
Dr Stephan Hofmann, who led the research, said: “In order to reach the full application potential for nanotubes, we need to be able to accurately control their growth first. As a manifestation of the impressive progress of nanometrology, we are actually now able to watch molecular objects grow. This new video footage shows that the catalyst itself remains crystalline but is constantly changing its shape as the carbon network is formed around it.
“We cannot yet solve the problem of not being able to self-assemble carbon nanotubes with well-defined characteristics, but we have discovered that if we are to do so, we need to be mindful not just of the carbon dynamics but the changing shape of the catalyst as well.”
Source: University of Cambridge
This news is brought to you by PhysOrg.com
IBM has jumped into water production & distribution. They currently have a web page that states:
Micromanaging our environment down to the nano-level
Five Innovations That Could Change Your Life in Five Years.
Early this year, IBM will undertake new research projects focused on the environment: advanced water modeling, water filtration via nanotechnology and efficient solar power systems.
Advanced water modeling, distribution and management systems
The ability to support economic and population growth has been contingent upon whether urban planners can ensure a reliable supply of water to residential and commercial establishments.
With the ubiquity of IP-based technology today, it is possible to envision a technologically enabled “smart” water distribution system that helps manage the end-to-end distribution, from reservoirs to pumping stations to smart pipes to holding tanks to intelligent metering at the user site so consumption could be managed in a responsible way.
The water distribution system would serve as a grid, much like a utility grid, at multiple levels: federal/central, regional, city/town and even down to a single residence or commercial establishment.
Water desalination using carbon nanotubes
The current methods of desalinating water, reverse osmosis and distillation, are both expensive and high maintenance. IBM will research methods of filtering water at the molecular level, using carbon nanotubes or molecular configurations, which can potentially remove the salt and impurities with less energy and money per gallon.Efficient solar power systems
Political instability, the high cost of fossil fuels and worries about global warming have increased interest in alternative energies. IBM is a leader in developing silicon technologies-the microprocessors that run the world’s leading game machines. We believe technology developments in this area will help further advance solar power and make it more efficient.
Why is IBM jumping into the Game. Many major companies are getting involved because the demand for water is rising faster than governments currently have solutions. This means the price of water will rise. Consider this Reuters Article:
ANALYSIS-Thirsty world captures investors’ attention
02 May 2007 11:00:41 GMT
By Christine Stebbins CHICAGO, May 2 (Reuters) – The competition for clean water is heating up and the world’s businesses have noticed.
The need to feed up to two billion more people by 2025, booming industrialization in developing countries like China, and a warming climate seen threatening the world’s most precious natural resource has investors serious about water.
“Regardless of what happens to the economy — you can bet and bank on a predictable demand for water. It is a product that is essential to life,” said Deane Dray, who analyzes water markets for Goldman Sachs in New York.
“People will largely pay ‘whatever’ because it is life-sustaining and there is no substitute. You put all those together, it is very clear why companies are enthusiastic about water.”
The United Nations Human Development Report for 2006 said that by 2025, if current global water consumption continues, more than 3 billion of the world’s 7.9 billion people will be living in areas where water is scarce.
Indeed, conflicts over water rights are already going on in dozens of areas from sub-Saharan Africa to the Middle East to Australia, India, eastern Asia and the U.S. Southwest.
One expert estimates that in the next 25 years trillions of dollars will be needed to upgrade fresh water and waste water technology and build new infrastructure to deliver water, with the bulk of that money to be spent in Asia.
“Infrastructure upgrades that are going to be required over the next 25 years on a global basis could be close to $20 trillion,” said John Balbach, managing partner at Cleantech Group, a venture capital research firm in green technology based in Ann Arbor, Michigan.
Such huge costs mean a budget nightmare for governments, a reality check that water companies also factor in. Eventually, they say, people in all countries will have to ration water use by price and realize it is not a free resource for the world.
“Governments globally are reaching a point where they’re not able to finance the delivery of cheap water, which is why the private sector is getting more and more interested,” said Balbach.
SKY’S THE LIMIT FOR REVENUES?
Global private industry sales in water-related sectors are estimated at $400 billion annually, including water infrastructure, treatment plants and new technologies to purify water. Of that total, $50 billion are bottled water sales.
Big investors seem most focused now in higher-tech segments of water companies including filtration, desalination and purification systems. But venture capital is also gravitating toward innovative solutions to costly problems.
California-based Underground Solutions Inc. slips pipes underground to repair leaky pipes that were installed more than 100 years ago without ever digging up city streets.
“Investments in water-related technology will go up by at least 50 percent this year,” said Nick Parker, Cleantech’s co-founder and chairman.
A recent Goldman Sachs report said it was likely, though, that over the next five years water system solutions will continue to be dominated by global giants including GE <GE.N>, Danaher <DHR.N>, ITT <ITT.N> and Siemens <SIEGn.DE>.
GE’s objective is to grow revenues by 8 percent every year “and we will definitely be north of that,” said Earl Jones, general manager of GE’s water and process technologies.
Dow Chemical <DOW.N> saw revenues from its water solutions group reach $450 million last year, more than double water revenues five years earlier. Dow also bought a Chinese engineering company, Zhejiang Omex Environmental Engineering Co., last summer in an acquisition aimed at water technology.
EVEN RICH GETTING POORER?
Agriculture and industry now account for roughly 80 percent of all water use, with the rest consumed by households.
But as industries and agriculture expand, the fight for and cost of water is likely to escalate, with pressure points seen rising in Asia, Australia and the Middle East, experts say.
Even in the United States, traditionally the world’s top food producer and exporter, is caught in the squeeze.
U.S. plans to cut dependence on foreign oil by switching to “green” fuels has ignited an industrial boom in the Midwest as ethanol and soy diesel plants spring up. But biofuel production consumes a huge amount of water, as do crops.
U.S. fresh water supplies are also shrinking.
The Ogallala, one of the largest underground U.S. aquifers, which runs from Nebraska to Texas, has seen water levels drop up to 30 feet in some spots in the last 10 years. A five-year old drought in the Corn Belt there also hasn’t helped.
Water levels in the U.S. Great Lakes, one of the largest pools of fresh water on the planet, are also dropping.
“It’s the most rapidly challenged critical resource in the world. It’s now almost a cliche: the 20th century was the century of oil and the 21st century will be the century of water,” said Henry Henderson of the New York-based Natural Resources Defense Council.
Desalination VS Water Transfers
26th April 2007
A couple weeks back I blogged about a widely published report that held that the west was entering into a prolonged drying spell. The New York Times detailed solutions being proposed & implimented that included desalination.
What was not mentioned was an idea that will be bandied about during a meeting in Calgary. That meeting will be held next week in Calgary. It addresses the idea of massive water transfers from Canada to the USA & Mexico to address water shortages. You won’t hear about it south of the border however. The only place this is mentioned is in Calgary.
April 25, 2007 April 25, 2007
Next week, government officials and academics from the three countries will gather in Calgary for the two-day North American Future 2025 Project (see page 6)where they’ll brainstorm ideas on how the continent should implement policies to deal with various challenges – including security, energy and labour.
But it’s the agenda on water that has activists concerned, given that the discussions will be held behind closed doors without public scrutiny, said Maude Barlow, national chairwoman of the Council of Canadians.
”We want this out in the light of day. We tried contacting them and they said this meeting is private,” Barlow said. ”How could it be private if it is setting up the political and policy framework for the future of North America?”
An outline of the proceedings states that climate change is expected to greatly exacerbate water shortages in the United States and Mexico while Canada, which has the world’s largest supply of fresh water in the Great Lakes and elsewhere, is not expected to suffer to the same extent.
It goes on to state that ”creative” solutions – such as water transfers and artificial diversions of fresh water – may be needed to address the ”profound changes” that are bound to occur south of the border.
Water transfers is something that’s hotly debated in Canada …(search google under Canada “bulk water”) but you don’t hear much about it in the lower 48–though President Bush has mentioned his support for the idea. Asked about the possibility of water transfers world renowned water expert Peter Gleick said the economics simply weren’t there. Mr. Gleick says.
I actually think this enormous controversy over bulk water exports is a little bit silly because no one’s going to be able to afford it,” he says.“And frankly I think some of these people who complain because they have been prohibited from doing it, I think we’ve saved them a lot of money. I think they should have been allowed to do it and go bankrupt.”
Santa Barbara looked into the idea several years back and decided on water desalination even at then current prices.
Never the less, according to a joint report entitled Global Water Futures produced by the CSIS and the Sandia National Laboratories.
Finding 5: Solutions must be innovative, revolutionary, and self-sustaining. Current
trajectories for improvement in freshwater availability and quality are inadequate to meet global
needs in a timely way. Innovative solutions must be found and employed that replace steady,
incremental rates of progress with dramatic, revolutionary changes. These solutions must be designed to be self-sustaining over the long-term.
Given the recognized urgency of the need for water solutions and the fact that the meetings are behind closed doors, it looks like much of the time & effort will be put into expediting Bush’s desire for water transfers–rather than doing any actual brain storming.
This is a shame. Especially as likely it will suck up what federal institutional energy there is behind water desalination R&D. Its especially shameful because the feds could get so much more bang for their buck out desalination R&D.
So if you happen to know someone who knows someone who is attending the meeting in Calgary next week…be sure to mention to them that basic research suggests that the cost of water desalination & transport will collapse in the next 5 to 10 years.
Here are three promising avenues of research mentioned in this blog from three different research labs.
Here’s a strategy for turning municipal sewage into pure water and oil.
Here’s a strategy for cutting the cost of pumping water
To hasten the pace of research, I would greatly increase the amount of money available to federal university & corporate labs for water desalination research. As well, I would include DARPA in the effort to fund start up companies. Further, I would suggest three ways to focus research dollars.
The first would be to make available prize money like the X-Prize that Newt Gingrich touts as a frugal way to get the most bang for the research buck. I blog about this in a piece called harvesting research unknown unknowns.
The second suggestion would be to attack known unkowns by employing a much less publicized method of crowdsourcing scientific research which I discuss in detail here.
How does a research administrator best deploy his dollars between projects competing for research dollars? Choosing rightly between known knowns is difficult. In fast paced industries companies use something called prediction markets. I discuss this strategy here.
Finally, make plain to those in attendance that those supporting Chinatown type scenarios are going to be overwhelmed and their careers sidelined by scientific innovation. In the next 20 years there will be more scientific innovation than the last 100 years. The best that the government can do is enable the scientists, the entrepreneurs and the corporations — and then sit tight. Water from Canada is nice but the right stuff comes from the ocean.
New RFP’s from the WateReuse Foundation
20th April 2007
There’s still a little time left for anyone interested in getting a hand in on these RFPs.
From Water and Wastewater.com
Industry News
New RFP’s from the WateReuse FoundationMar 29, 2007 – 2:25:22 PM
Alexandria, VA — The WateReuse Foundation announces the release of three new RFPs under its Solicited Research program. Proposals are to be submitted to the Foundation’s office in Alexandria, VA by 5:00 pm Eastern Time on May 2, 2007.
1) Low Cost Treatment Technologies for Small-Scale Water Reclamation Plants (WRF-06-008)
The overall goals of this project are to identify and evaluate established and innovative treatment technologies that will provide economic treatment processes that can be used in small-scale water reclamation plants, maximize automation to minimize labor requirements, increase treatment efficiency without sacrificing water quality, increase simplicity of operation, and increase the potential to export new treatment technologies to developing countries.
2) Predictive Models to Aid in Design of Membrane Systems for Organic Micropollutants Removal (WRF-06-009)
This project will improve and expand on one or more recently developed preliminary modeling techniques to predict the rejection of bioactive pharmaceutics and specific disinfection byproducts by RO membranes.
3) Guidance on Links Between Water Reclamation and Reuse and Regional Growth (WRF-06-016)
The objective of this project is to provide background and guidance to water reclamation and reuse managers and decision makers on connections between water reuse, water supply reliability, regional economic growth, demographic growth, and quality of life impacts for current residents.
The mission of the WateReuse Foundation is to conduct and promote applied research on the reclamation, recycling, reuse, and desalination of water.
For more information about submitting proposals to the Foundation: http://www.watereuse.org/Foundation/rfp.htm
Source: http://www.watereuse.org/
Vibratory Shear Enhanced Processing VSEP
13th April 2007
According to this article:
The Texas Water Development Board awarded San Antonio Water System a $205,000 grant to test a particular technology to turn brackish groundwater into high-quality drinking water.
SAWS will work with the Evergreen Underground Water District to churn highly salinated water into a potable water source for the region.
The research study will determine the feasibility and costs of Vibratory Shear Enhanced Processing technology at SAWS’ proposed desalination plant.
SAWS is an add on for membrane technology that prevents fouling. So one thing for membrane researchers out there to consider is that there are transitional ways around fouling like SAWS such that anti fouling need not be accomplished by the membrane itself. Below is the details for how SAWS works. (Come to think of it between SAWS and charge all you need from a membrane is flux.)
Products & Services – Vibratory Shear Enhanced Processing VSEP
[Technology] [System Operation] [System Components] [Applications] [Models] [Download]
While membrane-based separations of liquids from solids have enjoyed increasing popularity over the last 20 years, the technology has an inherent Achilles heel that affects all membrane devices: fouling. This long-term loss in throughput capacity is due primarily to the formation of a boundary layer that builds up naturally on the membranes surface during the filtration process. In addition to cutting down on the flux performance of the membrane, this boundary or gel layer acts as a secondary membrane reducing the native design selectivity of the membrane in use. This inability to handle the buildup of solids has also limited the use of membranes to low-solids feed streams.
 (Figure1)
(Figure1)
To help minimize this boundary layer buildup, membrane designers have used a method known as tangential-flow or cross-flow filtration that relies on high velocity fluid flow pumped across the membranes surface as a means of reducing the boundary layer effect. (See Figure 1)
In cross-flow designs, it is not economic to create high shear forces, thus limiting the use of cross-flow to low-viscosity (watery) fluids. In addition, increased cross-flow velocities result in a significant pressure drop from the inlet (high pressure) to the outlet (lower pressure) end of the device, which leads to premature fouling of the membrane that creeps up the device until permeate rates drop to unacceptably low levels.
 (Figure 2)
(Figure 2)
Instead of producing high cross flow, an alternative method for producing intense shear waves on the face of a membrane is developed. The technique is called Vibratory Shear Enhanced Processing (VSEP). In a VSEP System, the feed slurry remains nearly stationary, moving in a leisurely, meandering flow between parallel membrane leaf elements. Shear cleaning action is created by vigorously vibrating the leaf elements in a direction tangent to the faces of the membranes.
The shear waves produced by the membrane’s vibration cause solids and foulants to be lifted off the membrane surface and remixed with the bulk material flowing through the membrane stack. This high shear processing exposes the membrane pores for maximum throughput that is typically between 3 and 10 times the throughput of conventional cross-flow systems. (See Figure 2, above)
The oscillation produces a shear at the membrane surface of about 150,000 inverse seconds (equivalent to over 200 G’s of force), which is approximately 10 times the shear rate of the best conventional cross-flow systems. More importantly, the shear in a VSEP System is focused at the membrane surface where it is cost effective and most useful in preventing fouling, while the bulk fluid between the membrane disks moves very little.

Because VSEP does not depend on feed flow induced shearing forces, the feed slurry can become extremely viscous and still be successfully dewatered. The concentrate is essentially extruded between the vibrating disc elements and exits the machine once it reaches the desired concentration level. Thus, VSEP Systems can be run in a single pass through the system, eliminating the need for costly working tanks, ancillary equipment and associated valving.
The disc pack hold up volume of a system with 1,400 ft2 (130 sq. meters) of membrane area, is less than 50 gallons (189 liters). As a result, product recovery in batch processes can be extremely high.
An Arid West No Longer Waits for Rain
06th April 2007
Most newspapers this week have published reports as to the effect of global warming on deserts in the US southwest. Droughts will be longer and more persistant.
Perhaps in anticipation of the reports the New York Times this week did a piece on the water shortage out west that included methods for aleviating the drought. Water desalination is seen as one of many options to insure secure water supplies.
|
|
An Arid West No Longer Waits for Rain
A Western drought that began in 1999 has continued after the respite of a couple of wet years that now feel like a cruel tease. But this time people in the driest states are not just scanning the skies and hoping for rescue.
Some $2.5 billion in water projects are planned or under way in four states, the biggest expansion in the West’s quest for water in decades. Among them is a proposed 280-mile pipeline that would direct water to Las Vegas from northern Nevada. A proposed reservoir just north of the California-Mexico border would correct an inefficient water delivery system that allows excess water to pass to Mexico.
In Yuma, Ariz., federal officials have restarted an idled desalination plant, long seen as a white elephant from a bygone era, partly in the hope of purifying salty underground water for neighboring towns.
The scramble for water is driven by the realities of population growth, political pressure and the hard truth that the Colorado River, a 1,400-mile-long silver thread of snowmelt and a lifeline for more than 20 million people in seven states, is providing much less water than it had.
According to some long-term projections, the mountain snows that feed the Colorado River will melt faster and evaporate in greater amounts with rising global temperatures, providing stress to the waterway even without drought. This year, the spring runoff is expected to be about half its long-term average. In only one year of the last seven, 2005, has the runoff been above average.
Everywhere in the West, along the Colorado and other rivers, as officials search for water to fill current and future needs, tempers are flaring among competing water users, old rivalries are hardening and some states are waging legal fights.
In one of the most acrimonious disputes, Montana filed a suit in February at the United States Supreme Court accusing Wyoming of taking more than its fair share of water from the Tongue and Powder Rivers, north-flowing tributaries of the Yellowstone River that supply water for farms and wells in both states.
Preparing for worst-case outcomes, the seven states that draw water from the Colorado River — Colorado, Wyoming, Utah and New Mexico in the upper basin and California, Arizona and Nevada in the lower basin — and the United States Bureau of Reclamation, which manages the river, are considering plans that lay out what to do if the river cannot meet the demand for water, a prospect that some experts predict will occur in about five years.
“What you are hearing about global warming, explosive growth — combine with a real push to set aside extra water for environmental purpose — means you got a perfect situation for a major tug-of-war contest,” said Sid Wilson, the general manager of the Central Arizona Project, which brings Colorado River water to the Phoenix area.
New scientific evidence suggests that periodic long, severe droughts have become the norm in the Colorado River basin, undermining calculations of how much water the river can be expected to provide and intensifying pressures to find new solutions or sources.
The effects of the drought can be seen at Lake Mead in Nevada, where a drop in the water level left docks hanging from newly formed cliffs, and a marina surrounded by dry land. Upriver at Lake Powell, which is at its lowest level since spring 1973, receding waters have exposed miles of mud in the side canyons leading to the Glen Canyon Dam.
In California, Gov. Arnold Schwarzenegger has sounded alarm bells by pushing for a ballot measure in 2008 that would allocate $4.5 billion in bonds for new water storage in the state. The water content in the Sierra Nevada snowpack has reached the lowest level in about two decades, state hydrologists have reported, putting additional pressure on the nation’s most populous state to find and store more water.
“Scientists say that global warming will eliminate 25 percent of our snowpack by the half of this century,” Mr. Schwarzenegger said recently in Fresno, Calif., “which will mean less snow stored in the mountains, which will mean more flooding in the winter and less drinking water in the summer.”
In Montana, where about two-thirds of the Missouri River and half of the Columbia River have their headwaters, officials have embarked on a long-term project to validate old water-rights claims in an effort to legally shore up supplies the state now counts on.
Under the West’s water laws, claims are hierarchal. The oldest, first-filed claims, many dating to pioneer days, get water first, with newer claims at the bottom of the pecking order.
Still, some of the sharpest tensions stem more from population growth than cautionary climate science, especially those between Nevada and Utah, states with booming desert economies and clout to fight for what they say is theirs.
Las Vegas, the fastest-growing major city in the country, and the driest, developed the pipeline plan several years ago to bring groundwater from the rural, northern reaches of the state. The metropolitan area, which relies on the Colorado River for 90 percent of its water, is awaiting approval from Nevada’s chief engineer.
Ranchers and farmers in northern Nevada and Utah are opposed to the pipeline plan and have vowed to fight it in court, saying it smacks of the famous water grab by Los Angeles nearly a century ago that caused severe environmental damage in the Owens Valley in California.
“Southern Nevada thinks it can come up here and suck all these springs dry without any problems,” said Dean Baker, whose family’s ranch straddles the Nevada-Utah border, pointing out springs that farmers have run dry with their own wells. “We did this ourselves. Now imagine what pumping for a whole big city is going to do.”
Meanwhile, Utah has proposed a $500 million, 120-mile pipeline from Lake Powell to serve the fast-growing City of St. George and Washington County in the state’s southwestern corner. Nevada officials have said they will seek to block that plan if Utah stands in the way of theirs.
“Utah is being very disingenuous, and we’re calling them on it,” said Patricia Mulroy, the chief executive of the Southern Nevada Water Authority, the agency responsible for finding water for Las Vegas and its suburbs. “St. George, Utah, is growing as fast as southern Nevada, because the growth is going right up the I-15 corridor.”
Dennis J. Strong, director of the Utah Division of Water Resources, said Nevada was protesting too much and instead should be cheering the Lake Powell project because Colorado River water that Utah does not use would flow in Nevada’s direction. Mr. Strong said that Nevada’s protests “may be a bargaining chip.” He said he hoped for a compromise that would allow both projects to move forward.
In Yuma, near the Arizona border with Mexico, officials have pinned hopes on a desalination plant built 15 years ago. The plan then had been to treat salty runoff from farms before it made its way into Colorado River headed to Mexico, thus meeting the terms of an old water treaty.
But a series of unusually wet years made it more efficient to meet the treaty obligations with water from Lake Mead, so the plant sat idle. Drought has changed all that. Arizona water managers, who are first in line to have their water cut in a shortage under an agreement with other states, called for the plant to be turned on.
Under an agreement with environmentalists, the federal Bureau of Reclamation plans to monitor the environmental effects of using the plant, and study, among other things, using the purified water for purposes other than meeting its treaty obligations, like supplying the growing communities around Yuma.
“It never made sense to me to just dump bottled-water quality water into the river anyway,” said Jim Cherry, the bureau’s Yuma area manager.
What unites the Western states is a growing consensus among scientists that future climate change and warmer temperatures, if they continue, could hit harder here than elsewhere in the continental United States.
“The Western mountain states are by far more vulnerable to the kinds of change we’ve been talking about compared to the rest of the country, with the New England states coming in a relatively distant second,” said Michael Dettinger, a research hydrologist at the United States Geological Survey who studies the relationships between water and climate.
Mr. Dettinger said higher temperatures had pushed the spring snowmelt and runoff to about 10 days earlier on average than in the past. Higher temperatures would mean more rain falling rather than snow, compounding issues of water storage and potentially affecting flooding.
In some places, the new tensions and pressures could even push water users toward compromise.
Colorado recently hired a mediator to try to settle a long-running dispute over how water from the Rocky Mountains should be shared among users in the Denver area and the western half of the state. Denver gets most of the water and has most of the state’s population. But water users in the mountains, notably the ski resort industry, also have clout and want to keep their share.
Robert W. Johnson, the Bureau of Reclamation commissioner, said he shared the optimism that the disputes could be worked out, but he said he thought it might take a reconsideration of the West’s original conception of what water was for.
The great dams and reservoirs that were envisioned beginning in the 1800s were conceived with farmers in mind, and farmers still take about 90 percent of the Colorado River’s flow. More and more, Mr. Johnson said, the cities will need that water.
An agreement reached a few years ago between farmers and the Metropolitan Water District of Southern California, the chief supplier of water to that region, is one model. Under the terms of the agreement, farmers would let their fields lie fallow and send water to urban areas in exchange for money to cover the crop losses.
“I definitely see that as the future,” Mr. Johnson said.
Randal C. Archibold reported from Yuma, Ariz., and Kirk Johnson from Denver.
Smart thin film membranes adopt properties of guest molecules
30th March 2007
Consider this Craig Ventor piece about the new treasure trove of bacteria dna/proteins brought in by Craig Venter’s Institute. Science critics are hailing his work as the biggest deal in ocean going genetics since the Beagle voyages of Charles Darwin.
How would this effect desalination research. It would be helpful if someone in the desalination community tasked the Craig Ventor Institute to keep an eye out for proteins that do desalination work.
Great. So you get some desalination proteins. How do you make them into something useful?
Remember Ventor as you read the article below.
Public release date: 28-Mar-2007
Contact: Susan Trulove
STrulove@vt.edu
540-231-5646
Virginia Tech
http://www.eurekalert.org/pub_releases/2007-03/vt-stf031907.php
Smart thin film membranes adopt properties of guest molecules
Blacksburg, Va., March 28, 2007 — Virginia Tech researchers announced last year that they had created a nanostructured membrane that incorporates DNA base pairs in order to impart molecular recognition and binding ability to the synthetic material. This year they will show for the first time that these new films, membranes, and elastomers are compatible with diverse organic and inorganic molecules and will adopt properties of the guest molecules.
The research is being presented as an invited talk at the 233rd national meeting of the American Chemical Society in Chicago March 25-29.
Chemistry professor Tim Long’s research group, students affiliated with the Macromolecule and Interfaces Institute (MII) at Virginia Tech, and the U.S. Army Research Laboratory created a block copolymer, where different monomers are linked in a sequential manner and form a nanostructured film. They used adenine and thymine nucleotides, two of the four DNA base pairs that recognize each other. Then the researchers experimented with different kinds of guest molecules with complementary hydrogen bonding sites (hydrogen has a low energy attraction to many types of atoms).
The low energy attraction, means the guest molecules are widely dispersed throughout the membrane, which then takes on the properties of the guest molecules. “For example,” said Long, “if the guest molecules have ionic sites (sites with positive and negative charges), you will be able to transfer water through a film because you would have ion channels at the nanoscale. It’s similar to the way a cell membrane works to control the flow of specific ions into a cell. You can create protective clothing – against chemicals – that would still allow water vapor through.”
Salts, as ordinary table salt, are hydrophilic (water loving) and their introduction into a block copolymer template permits the placement of the salts at the nanometer dimension. One can imagine forming of channels of salts that are not visible with the human eye, but act as a roadway for the transport of water molecules.
“The research is synergy at the nanotechnology-biotechnology interface,” Long said.
###
The talk, “Nucleobase-containing triblock copolymers as templates for the dispersion of guest molecules at the nanoscale” (PMSE 423) will be presented at 9:05 a.m. Wednesday, March 28, in McCormick Place South room S505A. Authors are Brian Mather of Albuquerque, a chemical engineering doctoral student in MII; Margaux B. Baker, an undergraduate student from the University of Michigan who studied with Long’s group during summer 2006; Long, and Frederick L. Beyer of the U.S. Army Research Laboratory.
Mather received his undergraduate degree from the University of New Mexico. Learn more about his research at Virginia Tech by visiting www.chem.vt.edu/chem-dept/tlong/Brian.html.
![]() [ Print Article | E-mail Article | Close Window ]
[ Print Article | E-mail Article | Close Window ]

