Recent Posts
- Peace Through Water Desalination
- CPAC Water Policy Interview with KLRN Radio San Antonio Texas
- CPAC Water Interview With California Talk Show Host Rick Trader
- Toward a Green Earth Policy in the era of Trump
- Gates Foundation Water Energy Vision
Recent Comments
- on LLNL Researchers use carbon nanotubes for molecular transport
- on Greenhouses for Desalination
- on American Membrane Technology Association
- on Engineers develop revolutionary nanotech water desalination membrane
- on LLNL Researchers use carbon nanotubes for molecular transport
Archives
- May 2017
- March 2017
- June 2011
- December 2008
- November 2008
- October 2008
- September 2008
- August 2008
- July 2008
- June 2008
- April 2008
- February 2008
- January 2008
- December 2007
- November 2007
- October 2007
- September 2007
- August 2007
- July 2007
- June 2007
- May 2007
- April 2007
- March 2007
- February 2007
- January 2007
- December 2006
- November 2006
- October 2006
- September 2006
- August 2006
- July 2006
- June 2006
Categories
American Membrane Technology Association
27th July 2007
I’ve been in Las Vegas this week for an American Membrane Technology Association desalination conference. I’ll leave today for home haunts in Mclean, VA.
Flying in on Monday from the east coast the old desert valleys of western Utah and Nevada look like old dead lakes. Come to think of it — they are old dead lakes. Except there’s a blue tangle of finger lakes among the carved brown mountains to the south. These mark Lake Powell and Lake Mead. Man made lakes. Both are now half full.
There was a legislative breakfast on Wednesday morning. On the panel for the breakfast were Mike Connor, Counsel to the US Senate Committee on Energy and Natural Resources, Mike Gabaldon Director of Policy for the US Bureau of Reclamation and Mike Deane, Senior Policy Advisor for the EPA Office of Water. During the question period I went up to the audience mike and mentioned the experience of flying into Vegas — and seeing the man made lakes to the south. I said, “the man made lakes are great tributes to the vision and ambition of the generation of water men that produced them. Still those dams come at the end of a great period of technological innovation from +-1920-1930. As mentioned previously [at the breakfast], we are entering a simliar period of very fast innovation today. What grand vision today could we look forward to that would scale to the size of the vision of the great water men that produced such wonders as the Hoover dam back in the 1930’s.”
The question didn’t quite compute. Its hard to associate membranes and dams. I had some discussions afterwwards. People are mostly focused on the problems. The lakes are half full and people are still streaming into the southwest. Membrane development has been incremental for the last 30 years. Costs have come down but slowly and measured over decades and not, say, 18 month cycles like computer chips and more recently– photovoltaics. To do big water stuff requires money. The opinion of the conference generally was that there won’t be big money for desalination R&D until the lakes run dry. Then, after everyone has had their come-to-Jesus… moment–and the usual heads role–then there’ll be big money for desalination R&D.
Its better, of course, to focus on the opportunity rather than on the problems. That way, solutions come in a timely way. A stitch in time saves nine. A constant theme of this blog for the last year–based on publicly available information– has been that the research tools available today make it possible to collapse the cost of water desalination & transport by a factor of 10. Desalination & transport costs of 1/10th current costs– will make it economically possible to turn the deserts green, increase the habitable size of the USA by 1/3 and ultimately double the size of the habitable earth. With the right funding this research goal can be achieved in 10 years or less. 7-10 years from now instead of big coastal water desalination plants there would be pipes people stuck in the ocean that used the water itself as fuel to pump fresh water out and pumped it 1000 miles inland for costs comparable to east coast water. And the USA desalination tech would provide the template for desalination worldwide for the 21st century just as the Hoover Dam provided the template for the 20th century dam building worldwide.
I think that vision scales properly to the size of the vision of the 1930’s water men.
But is it doable? Consider. Newt Gingrich, a historian & a knowlegable washington establishment figure — has said repeatedly over the last year or two that there will be 4-7 times as much scientific change in the next 25 years as there was in the previous 25. According to Mr. Gingrich:
I used to say 4 times as much and then I gave a talk to the National Academy of Science’s working group on Computation and Information. And afterwards the Chairman said to me, “4 times isn’t big enough, it’s got to be at least 7.”
And where did the Chairman’s confidence come from? Actually, the federal government itself. The federal government is funding the research to enable super computers 10 years from now — to be 1000 times faster than today’s super computers–which are in turn 1000 times faster than super computers 10 years ago. It is not just the hardware. The software is moving to the point where it is now possible to model any kind of material you can imagine.–say a material that allows fresh water — but not salt in solution — to pass through a membrane at room temperature and pressure. Or maybe a cheap catalyst that seperates H2 from O at room temperature and pressure. Or maybe a catalyst in whose presence –salt simply settled out of solution. Or say a substance that easily/harmlessly binds to salt in solution to make a substance that’s profitable to sell or –whatever the designer wants. Same goes for pipes and pumps.
Finally, it bears mentioning that computers for the first time have given mathematicians an ever more powerful tool. The consequence is that we have entered a golden age for math— which presages ever more powerful tools.
Last year, I blogged about Mihail Roco, senior advisor for the nanotechnology to the National Science Foundation and a key architect of the National Nanotechnology Initiative. He penned a piece in the Scientific American. The article gives a roadmap for nano technology — which also provides a good context for projecting the future of desalination research. Mr. Roco discusses the stages of nano technology R&D.
The second stage, which began in 2005, focuses on active nanostructures that change their size, shape, conductivity or other properties during use. New drug-delivery particles could release therapeutic molecules in the body only after they reached their targeted diseased tissues. Electronic components such as transistors and amplifiers with adaptive functions could be reduced to single, complex molecules.
Starting around 2010, workers will cultivate expertise with systems of nanostructures, directing large numbers of intricate components to specified ends. One application could involve the guided self-assembly of nanoelectronic components into three-dimensional circuits and whole devices. Medicine could employ such systems to improve the tissue compatibility of implants, or to create scaffolds for tissue regeneration, or perhaps even to build artificial organs.
Notice Mr Roco didn’t say anything about membranes or cataylsts or anything desalination related? Why not? Mr Rocco was talking about where current funding is going. The federal government is already spending billions annually on nanotechnology to build the next generation industrial base for the USA. The key here to understand is that the tools are available to do the work and the scientists are eager for a big challenge. But they need direction and money.
Well we’ve talked about the vision thing.
That leaves money.
How much?
The australians looked at american research and decided it was worthwhile to invest 250 million over 7 years in water desalination research. Since the Australian GNP & Govenment budget is roughly 1/12 the size of the USA — a round number for a simliar USA project would be roughly 3 billion over 7 years. The Australians are looking to cut desalination costs in half in seven years–but they don’t have the big research labs and money that are available in the USA.
The model for the research program might be the original bureau of Reclamation membrane project that spent 1.4 billion in 2000 dollars over 30 years from the 1950’s-80’s. The model for the research project might also look something like the human genome project back in 1990. That project was funded for 3 billion dollars over 15 years. It took a bit over 10 to complete because of technological advancements and private sector tie ins.
For biotechnology –private sector tie ins came from Ventor Associates. In materials research it might come from IBM. As I posted in May, IBM has jumped into water production & distribution–as they see big research yields within five years. They currently have a web page that states: (click on Micromanaging the Future)
Advanced water modeling, distribution and management systems
The ability to support economic and population growth has been contingent upon whether urban planners can ensure a reliable supply of water to residential and commercial establishments.
With the ubiquity of IP-based technology today, it is possible to envision a technologically enabled “smart” water distribution system that helps manage the end-to-end distribution, from reservoirs to pumping stations to smart pipes to holding tanks to intelligent metering at the user site so consumption could be managed in a responsible way.
The water distribution system would serve as a grid, much like a utility grid, at multiple levels: federal/central, regional, city/town and even down to a single residence or commercial establishment.
Water desalination using carbon nanotubes
The current methods of desalinating water, reverse osmosis and distillation, are both expensive and high maintenance. IBM will research methods of filtering water at the molecular level, using carbon nanotubes or molecular configurations, which can potentially remove the salt and impurities with less energy and money per gallon.
Typically companies are much more willing to do basic & applied research if the federal government chips in half the funding. Its not just the money that moves private companies to fund their research departments. Its also the federal government leadership/imprimatur. (I give a more complete list of innovative funding techniques in this blog desalination VS bulk water transfer.)
IBM as well as other companies like GE would be good partners especially for investing in applied research to develop scalable (economical) processes for producing membranes that use nanotubes or other useful nanomaterials for desalination.
IBM will likely provide plenty of patent protection for their own work. But elsewhere the story may be different. imho any funding authority for research should come with some provision/money/help for deep patent protection for American Scientists–including filing patents overseas. The number of patents filed worldwide annually is going up by leaps and bounds. But most of the patents are derivitive. They link back to American patents–especially in the far East. The Japanese, Koreans and Chinese have learned to game the US patent system–with interlocking small patents. So its not unusual for the fruits of basic American research to go to foreign companies. This was especially the case for US membrane research from the 1950’s-80’s. That said, many unrelated US industries benefitted from US membrane research. The benefits of learning to specify and synthesize membranes, catalysts, pipelines and what have you — on the fly … will be orders of magnitude greater.
The nanostructures below from Sandia National Laboratories and the University of New Mexico (UNM), in conjunction with researchers at Case Western Reserve and Princeton Universities–may some day have some applicability to water desalination. I’m going to be at the membrane conference in Las Vegas next week 7/23-7/27. I’ll ask around about it.
![]()
Self-assembled nanostructures function better than bone as porosity increases

On the left is a TEM micrograph of a porous, cube-like nanostructure. On the right is a blow-up of the silica framework (the dark <2-nm thick regions on the left side figure) based on modeling. The highlighted structures represent the small rings referred to in the story. Credit: Sandia National Laboratory
Naturally occurring structures like birds’ bones or tree trunks are thought to have evolved over eons to reach the best possible balance between stiffness and density.
But in a June paper in Nature Materials, researchers at Sandia National Laboratories and the University of New Mexico (UNM), in conjunction with researchers at Case Western Reserve and Princeton Universities, show that nanoscale materials self-assembled in artificially determined patterns can improve upon nature’s designs.
“Using self-assembly we can construct silica materials at a finer scale than those found in nature,” says principal investigator Jeff Brinker. “Because, at very small dimensions, the structure and mechanical properties of the materials change, facile fabrication of stiff, porous materials needed for microelectronics and membrane applications may be possible.”
Nuclear magnetic resonance and Raman spectroscopic studies performed by Sandia researchers Roger Assink (ret.) and Dave Tallant, along with molecular modeling studies performed by Dan Lacks at Case Western Reserve University, showed that, as the ordered porous films became more porous, the silica pore walls thinned below 2 nm, re-arranging the silica framework to become denser and stiffer.
Whereas the stiffness of evolved optimized bone declines proportional to the square of its density, mechanical studies performed by Sandia researcher Thomas Buchheit working with UNM student Christopher Hartshorn showed that the stiffness/modulus of self-assembled materials was much less sensitive to increasing porosity: For a material synthesized with a cubic arrangement of pores, the modulus declined only as the square root of its density.
The silica nanostructures — basically a synthetic analogue of bone-like cellular structures, replicated at the nanoscale using silica compounds — thus may improve performance where increased pore volume is important. These include modern thin-film applications such as membrane barriers, molecular recognition sensors, and low-dielectric-constant insulators needed for future generation of microelectronic devices.
“Bone, closely examined, is a structured cellular material,” says Brinker, a Sandia Fellow and chemical engineering professor at UNM. “Because, using self-assembly, we had demonstrated the fabrication of a variety of ordered cellular materials at the nanoscale with worm-like (curving cylinders), hexagonal (soda straw packing) and cubic sphere arrangements of pores, we wondered whether the modulus-density scaling relationships of these nanoscale materials would be similar to the optimized evolved materials [like bone]. We found that both material structure and pore sizes matter. At all densities we observed that the cubic arrangement was stiffer than the hexagonal arrangement, which was stiffer than the worm-like. For each of these structures, increasing porosity caused a reduction in modulus, but the reduction was less than for theoretically optimized or naturally evolved materials due to the attendant stiffening of the thinning nanoscale silica walls resulting from the formation of small stiff silica rings.
“This change in ring structure only happens at the nanoscale,” says Brinker.
Sandia researcher Hongyou Fan created cubic, cylindrical, and worm-like (or disordered) pores to evaluate differences in stiffness resulting from these differently shaped internal spaces.
Other paper authors include Dave Kissel of UNM, Regina Simpson at Sandia, and Salvatore Torquato of Princeton.
Source: Sandia National Laboratory
water from air
29th June 2007
Back in 2001 the first reports came out on an african desert beetle that gathered water in its wings from the air.
 The beetle thrives in one of the driest places on Earth.
The beetle thrives in one of the driest places on Earth.
Last year some MIT scientists copied the structure of a beetle’s wings to make surfaces with hydrophilic/superhydrophobic patterning.
This year some Aussie’s developed a water from air collector. Once you look through the article article below, check out the follow up article–as well as the work of the MIT scientists mentioned above. There might be a way to significantly enhance the Australians work.
Now for my next trick, water from air
|
Monday, 25 June 2007 |
 |
Leaves and spiders’ webs beaded with dew have inspired a low-tech solution for collecting fresh water.
WatAir, an inverted pyramid made from elastic canvas, recycled polycarbonate, metal or glass, can reap dozens of litres of water a day from the air.
The inexpensive solution could help bring clean drinking water to people in remote or polluted areas, its developers say.
“The design has minimal special demands. It is low-tech and low-cost, and in fact can be even produced with local means,” says Joseph Cory, a PhD candidate at the Technion-Israel Institute of Technology and an architect at Haifa’s Geotectura Studio.
Cory and colleague Eyal Malka of Malka Architects recently won first place for the invention in a competition sponsored by WaterAid, an international nonprofit organisation dedicated to providing safe domestic water to poor nations, and Arup, a UK-based firm specialising in sustainable designs.
Cory and Malka were inspired by the passive way dew gathers on leaves, spiders’ webs, even on sleeping bags and tents.
They designed a four-sided structure shaped like an inverted pyramid, with each panel about 3 metres tall.
At night, dew drops bead up on both the tops and undersides of the panels. Because the dew collecting on top may contain dust, dirt or insects, that water could be used for irrigation. But dew from the underside is drinkable.
Gravity draws the drops downward into tanks, wells or bottles at the bottom.
A 96 square metre structure can extract a minimum of 48 litres of fresh water daily. But the dimensions can vary, says Cory, from a small personal unit that fills a water glass to several large-scale units that provide water for a community.
The low-tech approach requires only low-cost materials and is quick and easy to deploy, says Cory.
 |
WatAir can be built locally but is durable enough to be dropped by parachute from a plane.
The cost could be offset by printing sponsor logos or advertisements onto the canvas sheets.
“It is simple, practical, adaptable, sustainable, flexible and draws inspiration from nature resulting in a minimal intervention with potentially a big impact,” says Frank Lawson, a senior engineer at Arup.
Cory and Malka are also looking into modifications to WatAir that could help produce energy.
They are investigating embedding photovoltaic cells into the canvas to convert sunlight into electricity.
The energy could be used to power electrical appliances or charge batteries. Or it could be used to cool the surface of the dew panels, which would allow the structure to produce water all day long.
……………………………………….
Now consider the article below: Scientists at Ohio State have developed a kind of nano fiber that can attract or repel water. This fiber might be further enhance the device above. I’m sure the drawback here is that these fibers are much more expensive than the Aussie materials.
| New, invisible nano-fibers conduct electricity, repel dirt | |
|
A scanning electron microscope image of plastic fibers grown on a sheet of transparent film. Ohio State University researchers have invented a technique for carpeting a surface with tiny plastic fibers. The fibers can be made to attract or repel water and oil. Credit: Image courtesy of Ohio State University
Tiny plastic fibers could be the key to some diverse technologies in the future — including self-cleaning surfaces, transparent electronics, and biomedical tools that manipulate strands of DNA. |
|
In the June issue of the journal Nature Nanotechnology, Ohio State University researchers describe how they created surfaces that, seen with the eye, look as flat and transparent as a sheet of glass. But seen up close, the surfaces are actually carpeted with tiny fibers.
The patent-pending technology involves a method for growing a bed of fibers of a specific length, and using chemical treatments to tailor the fibers’ properties, explained Arthur J. Epstein, Distinguished University Professor of chemistry and physics and director of the university’s Institute for Magnetic and Electronic Polymers. “One of the good things about working with these polymers is that you’re able to structure them in many different ways,” Epstein said. “Plus, we found that we can coat almost any surface with these fibers.” For this study, the scientists grew fibers of different heights and diameters, and were able to modify the fibers’ molecular structures by exposing them to different chemicals. They devised one treatment that made the fibers attract water, and another that made the fibers repel water. They found they could also make the surfaces attract or repel oil. Depending on what polymer they start with, the fibers can also be made to conduct electricity. The ability to tailor the properties of the fibers opens the surface to many different applications, he said. Since dirt, water, and oil don’t stick to the repellant fibers, windows coated with them would stay cleaner longer. In contrast, the attracting fibers would make a good anti-fog coating, because they pull at water droplets and cause them to spread out flat on the surface. They devised one treatment that made the fibers attract water, and another that made the fibers repel water. They found they could also make the surfaces attract or repel oil. Depending on what polymer they start with, the fibers can also be made to conduct electricity. What’s more, researchers found that the attracting surface does the same thing to coiled-up strands of DNA. When they put droplets of water containing DNA on the fibers, the strands uncoiled and hung suspended from the fibers like clotheslines. Epstein said scientists could use the fibers as a platform to study how DNA interacts with other molecules. They could also use the spread-out DNA to build new nanostructures. “We’re very excited about where this kind of development can take us,” he added. Epstein’s research centers on polymers that conduct electricity, and light up or change color. Depending on the choice of polymer, the nano-fiber surface can also conduct electricity. The researchers were able to use the surface to charge an organic light-emitting device — a find that could pave the way for transparent plastic electronics. Finally, they also showed that the fibers could be used to control the flow of water in microfluidic devices — a specialty of study co-author L. James Lee, professor of chemical and biomolecular engineering and head of Ohio State’s Center for Affordable Nanoengineering of Polymeric Biomedical Devices. Lee and Epstein are advisors to former graduate student Nan-Rong Chiou, who developed the technology to earn his doctorate. He is now a visiting scholar at the university. Other co-authors on the paper included former doctoral students Chunmeng Lu and Jingjiao Guan. The technology is a merger of two different chemical processes for growing polymer molecules: one grows tiny dots of polymer “seeds” on a flat surface, and the other grows vertical fibers out from the top of the seeds. The fibers grow until the scientists cut off the chemical reaction, forming a carpet of uniform height. The university will license the technology, and Epstein and his colleagues are looking for new applications for it. Aside from anti-fog windows, self-cleaning windows, and organic LEDs, Chiou said that he foresees the surfaces working in glucose sensors, gene therapy devices, artificial muscles, field emission displays, and electromagnetic interference shielding.
Source: Ohio State University |
|
Advancing Desalination Technology.
22nd June 2007
The National Research Council’s (NRC) Water Science and Technology Board has undertaken a — Department of the Interior and U.S. Bureau of Reclamation — sponsored study on advancing desalination technology. They want to know how fast research is moving–ie how fast research will result in desal costs coming down. How much money to spend to make it happen. Where to allocate funds in the most promising research fields. How desalination compares to bulk water transfers. etc. Its been ongoing for about a year. It should be completed by year end. The last meeting is 08/08/2007.
If you want to participate and you don’t have private access –they will have some meetings open to the public. I’m thinking of going myself. But it would be better to have people who were closer to the research — throw in their two cents. And of course, for those whose research is dependent on federal dollars — Woods Hole, Mass August 8 — would be a good place to be. Come to think of it… Woods Hole has been a famous destination in years past for science people. So be there or be square.
Below is the only PR I’ve seen on this.
June 13, 2007
http://www.clarkson.edu/news/photos/zander.jpg .] 
Clarkson University Professor and Associate Dean of Engineering Amy K. Zander is the chair of the National Academy of Sciences Committee on Advancing Desalination Technology.
The National Research Council’s (NRC) Water Science and Technology Board has undertaken a study on advancing desalination technology. Zander’s committee is conducting a study that will produce recommendations to federal, state, and local governmental and private entities concerned with advancing desalination.
The committee will study the potential for both seawater and inland brackish water desalination to help meet anticipated water supply needs in the United States, assess the current state-of-the-science in desalination and recommend long-term goals for advancing desalination technology. Following up on an NRC recommendation calling for the development of a national research agenda, the committee will determine what research is needed to reach the long-term goals for advancing desalination and what technical barriers should be resolved with existing technologies.
The committee will also examine the practical aspects of implementation, like economics, financing, regulatory, institutional, public acceptance, and consider how much research funding is needed to significantly advance the field of desalination technology and the appropriate roles for governmental and non-governmental entities, including the private sector.
The study, sponsored by the Department of the Interior and U.S. Bureau of Reclamation, should be completed by the end of the year.
Zander has been a faculty member in the Department of Civil and Environmental Engineering at Clarkson since 1991. She was promoted to associate professor in 1997 and was named full professor in 2003. She has been the associate dean for Academic Programs in the Wallace H. Coulter School of Engineering since 2005.
Her research interests are in the areas of physical and chemical separations in environmental systems, especially drinking water and wastewater treatment technologies. Her work involves finding new solutions for safe drinking water and for minimal impact of water and wastewater treatment systems on the natural environment. She specializes in membrane processes — both pressure-driven and concentration-driven — in environmental processes.
Zander has published dozens of journal articles, written and co-written numerous book chapters, and delivered papers at some 50 professional and academic conferences throughout North America. She has managed research projects totaling over $800, 000 from the National Science Foundation (NSF), the American Water Works Association Research Foundation, and other funding agencies.
Zander has served on two prior committees of the National Academy of Sciences, producing the report Safe Water from Every Tap: Improving Water Service to Small Communities in 1997 and Confronting the Nation’s Water Problems: The Role of Research in 2004.
Her other honors include the Association of Environmental Engineering and Science Professors (AEESP) Distinguished Service Award in 2005; the 2003 Samuel Arnold Greeley Award from the Environmental and Water Resources Institute, a division of the American Society of Civil Engineers (ASCE); the AEESP/McGraw Hill Award for Outstanding Teaching in Environmental Engineering and Science; and Clarkson’s 1999 Distinguished Teaching Award.
The National Academy of Sciences (NAS) is an honorific society of distinguished scholars engaged in scientific and engineering research, dedicated to the furtherance of science and technology and to their use for the general welfare. The NAS was signed into being by President Abraham Lincoln in 1863, at the height of the Civil War. As mandated in its Act of Incorporation, the NAS has, since then, served to “investigate, examine, experiment, and report upon any subject of science or art” whenever called upon to do so by any department of the government.
Clarkson University, located in Potsdam, New York, is a private, nationally ranked university with a reputation for developing innovative leaders in engineering, business, the sciences, health sciences and the humanities. At Clarkson, 3, 000 high-ability students excel in an environment where learning is not only positive, friendly and supportive but spans the boundaries of traditional disciplines and knowledge. Faculty achieves international recognition for their research and scholarship and connects students to their leadership potential in the marketplace through dynamic, real-world problem solving.
Find out more about the study at http://www8.nationalacademies.org/cp/projectview.aspx?key=48674.
[News directors and editors: For more information, contact Michael P. Griffin, director of News & Digital Content Services, at 315-268-6716 or mgriffin@clarkson.edu.]
Tunnel Borers
15th June 2007
According to this article there are 20 desalination plants on the drawing boards in California. The costs described by the article look like 1990’s costs. These costs have been more than halved in the last decade or so. A recurring problem suggested by the article is intake structures. See the article below on how the Australians are dealing with intake structures.
I really like the picture below. See how the Australians solve the problem of wee beasties harmed by salt concentrate.
The bore arrives at the desalination plant this morning.
Tunnel borers arrive for Tugun desal plant
Tony Moore | May 31, 2007 – 2:30PM
The first of two German-built tunnel boring machines to be used at the Tugun Desalination Plant has arrived at Tugun to begin the tunnel “under the seabed”.
The two laser-guided machines, which utilise GPS technology, will dig the inlet and outlet tunnels for the seawater to be used in Queensland’s first desalination plant.
At Tugun, just to the north of Coolangatta Airport, two 70-metre vertical tunnels have been built to allow the project team to build two horizontal tunnels which extend about 1.5 kilometres out to sea.
Infrastructure Minister Anna Bligh said the Tugun project was on track to provide 125 megalitres of desalinated water by the end of November 2008.
“This project is critical to beating the drought and they (the workers) know it,” she said.
“This tunnel is being (worked on) 24 hours a day. This project is on track to meet its scheduled completion date of November 2008.”
The Queensland Water Commission project reports for April show the project will provide water at “33 per cent capacity” by November 2008, and “water at 100 per cent capacity” by January 15, 2009.
Ms Bligh said the Tugun project had several advantages over desalination projects elsewhere in Australia.
“A great benefit of the Tugun site is that unlike Sydney and other places, this is a marine tunnelling project, having minimal impact on the environment and local communities as the tunnels – which will be 70 metres underground – do not run under any privately-owned land,” she said.
Eleven kilometres of pipeline to connect the desalination plant to the Western Corridor Recycled Wastewater Project have been delivered.
Ms Bligh said the progress at Tugun did not mean the government was still looking at a concept for a desalination plant on Bribie Island.
“The issues in relation to possible other locations for desalination plants are quite complex,” she said.
“Every location has its own challenges. The issue with Bribie Island is that it is located close to a very shallow and important marine ecosystem – and that is Moreton Bay.
“Here at Tugun we can take the water about 1.5 kilometres out to the deep ocean where the brine can be distributed without any damage to the marine environment.
“Moreton Bay is a very sensitive fish and marine habitat. It is much more shallow and we are very, very hesitant about putting a desalination plant into that environment.
“There are no plans on our books for a desalination plant at Bribie Island.”
………………………
I mention drilling underwater in passing in an earlier at the end of an awkwardly named blog
California Solar’s Revolutionary Energy Business Model for Desalination Pumps
A study group priced the drilling at 2 million. But the length of the intake tunnels is likely 200 yards rather than 2000 yards as is the case in Australia.
Low Pressure Desalination
08th June 2007
If you read last weeks blog Saltwater into Fire any time between last Friday and Tuesday–you’ll want to check back. I have been pretty steadily updating it. Now it appears that unlike electrolysis –which is net negative for energy output–low energy RF is net positive for energy output. imho that’s cool. (uh, well, actually, world beating.) However, the secret sauce that makes this work imho is even cooler. I think you’ll find this to be interesting too. Check it out.
Things can and do move quickly. In January I blogged about low pressure desalination. The blog discussed the research results of some Florida scientists. Now six month later the first prototypes based on that work have come out. (When you finish this article you might ask yourself why not burn saltwater & use it as a heat source. well why not? … It might be too hot)
Eye on Research: Researchers develop low-cost, low-energy desalination process
Sun News Report
Las Cruces Sun-News
Article Launched:05/27/2007 12:00:00 AM MDT
NMSU report
A low-cost water desalination system developed by New Mexico State University engineers can convert saltwater to pure drinking water on a round-the-clock basis and its energy needs are so low it can be powered by the waste heat of an air conditioning system.
A prototype built on the NMSU campus in Las Cruces can produce enough pure water continuously to supply a four-person household, said Nirmala Khandan, an environmental engineering professor in NMSU’s Department of Civil Engineering.
New Mexico and other parts of the world have extensive brackish groundwater resources that could be tapped and purified to augment limited freshwater supplies, but traditional desalination processes such as reverse osmosis and electrodialysis consume significant amounts of energy.
This research project, funded by the NMSU-based New Mexico Water Resources Research Institute, explores the feasibility of using low-grade heat — such as solar energy or waste heat from a process such as refrigeration or air conditioning — to run a desalination process.
Khandan said the project builds on a process, first developed by researchers in Florida, that makes distillation of saline water possible at relatively low temperatures — 113 to 122 degrees Fahrenheit rather than the 140 to 212 F required by most distillation processes.
The system utilizes the natural effects of gravity and atmospheric pressure to create a vacuum in which water can evaporate and condense at near-ambient temperatures. Two 30-foot vertical tubes — one rising from a tank of saline water and the other from a tank of pure water — are connected by a horizontal tube. The barometric pressure of the tall water columns creates a vacuum in the headspace.
At normal temperatures, Khandan said, evaporation from the pure-water side will travel to the saline side and condense as the system seeks equilibrium. “That’s nature,” he said. “We want it to go the other way.”
Raising the temperature of the water in the headspace over the saline column slightly more than that of the freshwater column causes the flow to go in the other direction, so that pure, distilled water collects on one side and the brine concentrate is left behind in a separate container. A temperature increase of only 10 to 15 degrees is needed, Khandan said.
“That’s the trick of this vacuum,” he said. “We don’t have to boil the water like normal distillation, so you can use low-grade heat like solar energy or waste heat from a diesel engine or some other source of waste heat.”
Potentially a desalination system using this method could be coupled to a home’s refrigerated air conditioning system, Khandan said.
“When you air condition a house, you are pumping the heat outside the house, and the heat is wasted into the atmosphere,” he said. “We want to capture that heat and use it to power this desalination system.”
The 30-foot-tall NMSU prototype is powered by a solar panel. Khandan and his research assistant, civil engineering doctoral student Veera Gnaneswar Gude, have modified the process originally developed by Florida researchers to incorporate a thermal energy storage device that allows the system to operate around-the-clock, using stored energy at night. The Institute of Energy and Environment housed in the NMSU College of Engineering helped them instrument the system.
Their research on the system’s capabilities has been presented at national and international conferences and their research continues.
As with any desalination process, the system leaves behind a brine concentrate that must be disposed of, and some potential users may be put off by the unit’s height, “but this technology could go to commercial scale pretty quickly,” Khandan said. “The overall cost of desalination by this process can be very competitive.”
The project is one of many research initiatives at NMSU aimed at addressing the critical needs of New Mexico and the nation.
“Eye on Research” is provided by New Mexico State University. This week’s feature was written by Karl Hill of University Communications.
Saltwater into fire
01st June 2007
You really have to see this to believe it. No that’s likely not good enough. You’ll have to get your own low energy radio wave machine and toast some salt water yourself. That’s what’s John Kanzius has done. The Sanibel Island Florida inventor was looking for a way to desalinate water but instead found a way to burn salt water. According to reports low energy radio waves split the H20 up into hydrogen & oxygen. It looks like The Na & Cl in solution acted as a heat sink or electrolyte . Or anyhow I assume so since the process wouldn’t work on fresh water. Maybe the RF acts as the catalyst as this discussion suggests. See below for the answer. One way or another -something bubbled out of solution. And with a flick of a bic the gases turned to fire.
Anyhow here’s a couple videos.
Salt Water into Fuel
Saltwater into fire 2
Saltwater into fire 3
Saltwater into fire 4
Understand. Electrolysis results in a net loss of energy. ie you put in more energy than you get out. Do not conflate electrolysis with radio waves. Radio waves represent a much smaller energy input.
Small enough to make for a net gain in energy?
For anyone with serious math skills, it should be possible to do a rough–back of the envelope– calculation based on the comments of this article:
Charles Rutkowski placed a test tube filled with ordinary salt water into John Kanzius’ external radio-wave generator.
He then blasted the salt water with 200 watts’ worth of directed radio waves, not quite enough electricity to light three 75-watt light bulbs.
Within seconds, a blue flame erupted from the top of the test tube. It then turned bright white like a blowtorch’s flame and burned for several minutes at about 3,000 degrees Fahrenheit.
“I’ve done this countless times and it still amazes me,” said Rutkowski, general manager of Industrial Sales and Manufacturing, the Millcreek company that builds Kanzius’ generators.
So what’s going on?
For starters, here’s a simple experiment that shows that salt increases many fold the output of hydrogen from electrolysis. (The Kansius experiment does not involve electrolysis but rather Radio Waves).
Here is a second bit of fun with a microwave that shows that the microwave can turn a flame off and on at will and make the flame burn high & hot. You can see in Kansius’s salt water burning video how Kansius turns the high hot fire off and on at will. The suggestion here is that the radio waves are both splitting the water and then further exciting the flames. As well, I’m suggesting that the process is analogous to that in a microwave oven.
Update:I talked on the phone to one of the scientists Ed Apsega at APV Engineering in Akron Oh. They tested John Kanzius process. I was told the flame burned at more than 1700 degrees Celcius or 3000 degrees Farenheit. (Its not clear to me currently as to whether the energy yield is more or less than 1:1. Why? Well the APV engineering scientist Ed Apsega said the energy yield was much more than 2:1 and later I talked to John Kanzius–he said the energy yield was less than 1:1.) The yellow flame was the glass burning. (The flame started out clear.) The temperature inches away from the flame was room temperature. Tests afterwards showed that the water was reduced and the mineral content by percent increased in the water. The Na in the water decreased but not significantly. John Kanzius would like government money for his salt water project so that he can work on his cure for cancer. I’ve been promised a follow up email/phone call from Kanzius so that he can provide a contact number/email that I can post. So check back.Update: Ok I talked with John Kanzius. He’s ok with being reached at johnkanzius (at) aol.com. There are three machines available. It would be helpful if someone qualified checked this thing out.
According to this site:
June 01, 2007
“Regarding moving this forward, I want to see what are the best results we can achieve with joules in vs joules out. A chemist in Houston whom I know is going to be doing a couple of things for me this weekend.” — John Kanzius (June 01, 2007)
“What burns at a temperature of over 1700 C? [Knowing the answer to that question] might take some of the guess work out of the equation.” (May 29, 2007)
June 06, 2007
John Kanzius writes:
“Since it appears we now have now achieved more than unity, I am going to do an embargo on releasing all further information.
“Actually there are smart individuals who have posted on different web sited and actually have a pretty good idea of what is happening.”
……………….
So why is the flame so high? Why doesn’t the wick burn? Why does the temperature so near to the flame revert back to room temperature? (See below.) The answer to all three questions seems to be that the flame shown in the video is an electrical fire. Some are calling it a plasma fire.
Slide Three of this slide show has a still of the Therm Med LLC External RF System. The machine is proprietary radio frequency machine. You’ll have to check with John Kanzius about that. See above.It appears that the secret sauce in the process is in the RF frequency. The frequency itself is the catalyst.
Consider this patent on the process: (It looks like somehow the radio waves immitate platinum.)
Catalytic simulation using radio frequency waves
Document Type and Number:
United States Patent 6217712
Link to this page:
http://www.freepatentsonline.com/6217712.html
Abstract:
The invention relates to a method of using radio frequency waves to artificially create catalytic action in a catalyst-free chemical reaction within a substance. To mimic or imitate the catalyst, radio frequency waves are transmitted through the substance at a signal strength sufficient to electronically reproduce the effect of the physical presence of a selected catalyst. The radio frequency waves have a selected transmission frequency substantially equal to a catalyst signal frequency of the selected catalyst, defined as the signal frequency determined by nuclear magnetic resonance of the selected catalyst. It is commonplace to use nuclear magnetic resonance to identify elements within a substance and the signal frequencies of various elements (including catalysts) are listed in widely published tables. To date, the mechanism by which catalysts bring about chemical reactions has been unknown. The inventor has recognised that the physical presence of a catalyst brings about a chemical reaction due to the emission of low intensity radio frequency waves from the catalyst with the signal frequency that is emitted being the signal frequency of the catalyst that is commonly determined by nuclear magnetic resonance. Therefore, the invention can be used to eliminate the need for expensive metallic catalysts, such as platinum. The invention electronically reproduces the effect of the physical presence of a catalyst by transmission of a radio frequency wave with a signal frequency equal to that signal frequency emitted by the catalyst and as determined by nuclear magnetic resonance of the catalyst.
Here is a list of his patents related to the process.
more on patents:
download PDF (Systems and methods for combined RF-induced hyperthermia and radioimmunotherapy, 2005)
download PDF (Enhanced systems and methods for RF-induced hyperthermia II, 2006)
European Patent Office (Kanzius patents)
WIPO.int (Kanzius patents)
European Patent Office RASBACH KLAUS (DE) This is an expired patent. I believe this gives the key.
In resonance-based generation of H2 and O2 from water, using a hypersonic generator of suitable frequency, the resonance frequency (fO) can be that corresp. to the distance (d) between the nucleus of the O atoms and its outer electron shell or the proton. fO can be calculated approx. from the formula: fO = c/(pi.d), where c= the speed of sound in water and pi= the Ludolf’s no). USE/ADVANTAGE – The H2 can be used as fuel in power stations or in hydrogenation (hardening fat), synthesis of petrol, MeOH and NH3, redn in metallurgy, in welding etc. The O2 can also be used for technical and other purposes. The overall efficiency of the process is much higher than usual and the process is more friendly to the environment.
According to this poster:
“Perhaps if we could find a substance with a NMR frequency of 13.56 mhz then that is our catalyst…”. Now if you can just turn that around a bit–you might get that the frequency that the machine is imitating is that produced by platinum or 13.56 mhz.
According to this poster:
From what I understand of this, (1) day reading, is that every metal has a moleculer frequency. The patent says that these numbers are readily available.
Next, you take your RF signal generator and set it to that frequency and put it into the solution according to the patent.
The solution “feels” the catalyst. If it had a brain it would be tricked into thinking it is seeing that particular metal.
My first thought is KOH in water and Aluminum. Replace the Aluminum with the molecular resonant frequency of the Aluminum metal and you have the KOH water mixture thinking you just put in AL. And here comes Hydrogen!! Can you say,”unbelievable!” Where has this tech been?!
Here are additional links:
Fla. Man Invents Machine To Turn Water Into Fire
How John Kanzius’ push to cure cancer may have discovered alternate fuel
Here’s a google search for Kanzius+burn+water.
“On our way to try to do desalinization, we came up with something that burns, and it looks in this case that salt water perhaps could be used as a fuel to replace the carbon footsteps that we’ve been using all these years, i.e., fossil fuels,” Kanzius said.
If it’s for real, the possible ramifications of the discovery are almost mind-boggling, as cars could be fueled by salt water instead of gasoline, hydroelectric plants could be built along the shore, and homes could be heated without worrying about supplies of oil.
“It doesn’t have to be ocean salt water,” Kanzius said. “It burns just as well when we add salt to tap water.”
Thus far, Kanzius’ work has not received extensive national publicity, but has been featured on several local television news programs, including WPBF-TV in West Palm Beach, Fla., WSEE-TV in Erie, Pa., and WKYC-TV in Cleveland. “We discovered that if you use a piece of paper towel as a wick, it lights every single time and you can start it and stop it at will by turning the radio waves on and off,” Kanzius told the Times-News as he watched a test tube of salt water burn.
“And look, the paper itself doesn’t burn,” he added. “Well, it burns but the paper is not consumed.”
Kanzius said he hasn’t decided whether to share his fuel discovery with government or private business, though he’d prefer a federal grant to develop it.
uh…. can someone fit this man’s work into their budget? I’m sure this could be worked around for water desalination/transport purposes.
Another but dissimilar process was announced recently where an aluminum-gallium alloy acted as the catalyst: (ie no RF was needed to make the reaction. But you might be able to tune radio wave to immitate the RF of aluminum-gallium)
I have blogged about working on desalination catalysts before but not really from the angle approached here. You can do a quick search on google for RF +deposition OR RF “water splitting” OR RF semiconductor. It looks like the semiconductor industry uses RF energy to split gas molecules, then recombines them to deposit chemical films onto wafers. According to this poster
I’ve seen another piece of equipment which uses magnetic
fields to direct ionized gases. I imagine this might be one method to
harvest the hydrogen before it has a chance to recombine with oxygen.
You might also tune RF to settle Na & Cl ions out of solution. Just a thought.
Last month the New York Times published an article about how the West is likely entering a prolonged period of water shortages. Similiar reports have recently been published in Australia detailing expected extended droughts over the next 50 years.
The USA and Australia have responded to these reports in different ways.
Several weeks ago I blogged about the current administration’s effort to push bulk water tranfers from Canada. This week Australia announced they were about to embark on a major desalination research project with the view of spending $250 million over seven years and cutting energy costs for desalination in half. Interestingly, their belief that such results are doable is based on recent research in the US. Further, they considered long distance bulk water transfers. They concluded, however, that doing the research to lower the cost of desalination was less expensive and complicated. And too, the ocean is a more reliable resource. See my concluding remarks after the article posted below.
Thirsty Australia Advances Desalination Technology
MELBOURNE, Australia, May 18, 2007 (ENS) – The delivery of energy efficient water desalination to drought-stricken Australia received a boost today with the establishment of a new collaboration between the government research agency CSIRO and nine Australian universities.
The research aims to advance water desalination as an alternative water supply option for Australia by increasing efficiency, and reducing the financial and environmental costs of producing desalinated water.
Australia, especially southern Australia, is short of water, and the country is experiencing the worst drought on record this year. Desalination of seawater is a possible additional supply, but it requires a lot of electricity, and is expensive, costing about A$1.10 per 1,000 liters (US$.90 per 264 gallons).
The new research effort, known as the Advanced Membrane Technologies for Water Treatment Research Cluster, is led by Professor Stephen Gray of Victoria University.
As a first step, the multi-disciplinary research team will carry out an evaluation of existing membranes and develop new energy efficient membranes.

Professor Stephen Gray is director of the Institute of Sustainability and Innovation at Victoria University, where he is responsible for research, education and industry liaison in the water, energy and sustainable buildings sectors. (Photo courtesy ISI)
“Many desalination and recycling programs rely on a process called reverse osmosis, where the water is forced through a semi-permeable membrane, removing salts and any other contaminants,” Gray explains.”These membranes need regular replacement and cleaning, but they also require a large amount of energy to force water through what are nano-sized pores,” he says.
When contaminants such as salts are removed from water, some of them adhere to the surface of the membrane, building up on the surface, increasing the pressure and energy required to desalinate the water.
“Chemicals are used to clean the membranes, but membrane surfaces that are less sticky would reduce the pressure and energy required and the frequency of cleaning,” Gray says.
The researchers aim to improve membrane anti-fouling properties, increasing the ability of the membranes to clean themselves without chemicals.
The research will link with and inform related CSIRO research into membrane and carbon nanotube water filtration technologies.
Carbon nanotubes, molecules made of carbon atoms, are hollow and more than 50,000 times thinner than a human hair. Billions of these tubes serve as the pores in a desalination membrane.

Carbon nanotubes can be made in many different configurations. (Photo courtesy Softpedia)
The smooth inner walls of the nanotubes allow liquids and gases to rapidly flow through, while the miniscule pore size keeps out larger molecules.Alan Gregory, urban water research leader at CSIRO, says, “In combination with other research projects led by CSIRO, we aim to reduce by up to 50 percent the amount of energy required to desalinate seawater using membranes. This same technology will have benefits for the treatment and recycling of wastewater.”
CSIRO researchers are using nanotechnology to develop a new membranes for desalination with electrodialysis technology, which they say may lead to breakthrough technologies in cost-effective and highly efficient water recovery systems.
Nanotechnology for water desalination is a rapidly developing field. In the United States, researchers at Lawrence Livermore National Laboratory announced in May 2006 their creation of a membrane made of carbon nanotubes and silicon that may offer less expensive desalinization.
The CSIRO scientists are developing new “inorganic-organic nanocomposite membranes for desalination by electrodialysis membrane process, which involves the incorporation of oxide nanoparticles into ion-conducting polymers to form new nanocomposites.”
“This also means we could potentially provide more secure water supplies while minimizing greenhouse gas emissions,” said Gregory.
Other partners in the membrane research program are the University of New South Wales, Monash University, the University of Melbourne, RMIT University, Curtin University of Technology, the University of Queensland, Deakin University, and Murdoch University.
Funding for the research was announced by Minister for Education, Science and Training Julie Bishop under the Flagship Collaboration Fund.
Desalination membrane advances cannot come soon enough for Australia, which is opening giant desalination plants already based on existing membrane technology, even if the water they produce is costly.

The new Perth Seawater Desalination Plant, shown here under construction, is the largest desalination plant in the southern hemisphere. (Photo courtesy ABB)
In April, the Water Corporation of Western Australia opened the 45 gigaliter Perth Seawater Desalination Plant. The US$290 million project will guarantee 17 percent of Western Australia’s current water needs, regardless of rainfall or drought.On Tuesday Western Australia Premier Alan Carpenter announced that a second desalination plant of the same size would be built at Binningup.
Meanwhile, the New South Wales Government of Premier Morris Iemma is moving forward with a huge desalination plant south of Sydney at Kurnell. The plant will use reverse osmosis technology with membranes that remove salts and other impurities from seawater to produce drinking water.
The environmental assessment for the construction and operation of a pipeline for Sydney’s desalination plant is open for public comment to Monday May 28.
As part of the desalination project, an 18 kilometer pipeline will be constructed from Kurnell, across Botany Bay, to Erskineville.
Sydney Water Managing Director Kerry Schott said the Kurnell plant would be 100 percent powered by green energy and would guarantee Sydney’s water supply.
“Given the uncertainty of climate change and Sydney’s growing population, alternative sources of water need to be developed,” said Schott.
“The desalination plant will supply about seven percent of Sydney’s water supply by 2009 but it can be scaled up further if required,” he said. “This gives us a supply of water that does not depend on rainfall.”
…………………………………………………………………………………….
I blogged last December about the LLNL scientists visit to Australia. Every provincial newspaper in Australia had a write up on that visit. This contrasts sharply with the notice that was given to the work of the LLNL scientists in the USA. Their write ups were mostly confined to science journals. Perhaps that’s why the Bush administration is actively considering bulk water transfers rather than accelerating the pace of desalination research. The US political class simply hasn’t been told whats going on in the US labs. Its not that the info isn’t available. Unlike a year ago,the implications of current research has pushed into corporate America. Two weeks ago I posted that IBM was entering into membrane research in the belief that great strides would be made in the next five years.
More to the point, as in the USA –the Australians considered pumping water over great distances and mountains and concluded that water desalination research was the better alternative. Consider this article.
Saltwater offers best hope, says scientist
Desalination and an inland pipeline are two of the options being considered by the State Government as it grapples with Melbourne’s water shortage.
Pumping water over the Great Dividing Range would probably be as energy intensive as desalination, he said, but the supply would be less reliable.
“We believe we can significantly reduce the amount of energy needed for desalination and this will make it even more competitive,” he said.
Its a shame sober men can’t come to the same conclusions in the USA.
Carbon nanotubes to the rescue of Moore’s law
18th May 2007
One big problem currently with using carbon nanotubes is producing them in high volumes at low prices. Luckily carbon nanotubes can be used for an infinite variety of purposes. (imho carbon nanotubes will wind up being what steel was to the 19th century and plastics was to the 20th century.) Because of this great diversity of applications a lot of different industries are attacking the problem of producing carbon nanotubes in high volume at low prices. For this reason desalination membrane researchers and administrators need to look at how other research disciplines and industries are grappling with producing carbon nanotubes cheaply in volume–with an eye out to adapting their processes to making carbon nanotube desalination membranes.
Below is an article about work in Germany being done to make computer chips in volume at cost from carbon nanotubes. imho it makes for interesting reading.
May 16, 2007
Nanowerk LLC
Carbon nanotubes to the rescue of Moore’s law
(Nanowerk Spotlight) Over the next few years, semiconductor fabrication will move from the current state-of-the-art generation of 90 nanometer processes to the next 65 nm and 45 nm generations. Intel is even already working on 32 nm processor technology, code-named “Westmere”, that is expected to hit the market sometime around 2009. The result of these efforts will be billion-transistor processors where a billion or more transistor-based circuits are integrated into a single chip. One of the increasingly difficult problems that chip designers are facing is that the high density of components packed on a chip makes interconnections increasingly difficult. In order to be able to continue the trend predicted by Moore’s law, at least for a few more years, researchers are now turning to alternative materials for transistors and interconnect and one of the prime candidates for this job are single-walled carbon nanotubes (SWCNT). However, one of the biggest limitations of conventional carbon nanotube device fabrication techniques today is the inability to scale up the processes to fabricate a large number of devices on a single chip. Researchers in Germany have now demonstrated the directed and precise assembly of single-nanotube devices with an integration density of several million devices per square centimeter, using a novel aspect of nanotube dielectrophoresis. This development is a big step towards commercial realization of CNT-based electronic devices and their integration into the existing silicon-based processor technologies.
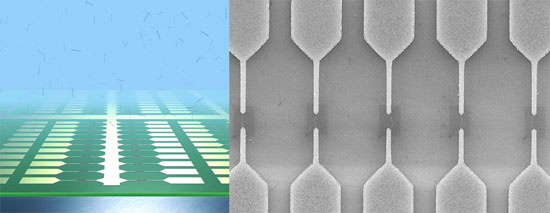
The image on the above shows a schematic of an ultra-large scale array of single-walled carbon nanotube devices fabricated by dielectrophoretic deposition from an aqueous solution. The scanning electron micrograph on the right shows the zoom-in to one region of the array showing each electrode pair bridged by an individual carbon nanotube in a self-limiting mechanism. (Images: Dr. Vijayaraghavan, Dr. Krupke, Forschungszentrum Karlsruhe)
“The fundamental issue of CNT device fabrication remains the biggest challenge for effective commercialization of nanotube electronics” Dr. Ralph Krupke explains to Nanowerk. “For CNT electronics to become a reality, it should be possible to scale up the fabrication technique to simultaneously and reproducibly fabricate a very large number of such devices on a single chip, each accessible individually for electronic transport. Conventional nanotube growth and device fabrication techniques using chemical vapor deposition or spin-casting are unable to achieve this, due to a lack of precise control over nanotube positioning and orientation.”
“Since these nanotubes are usually grown at temperatures greater then 500°C and show no growth selectivity between metallic and semi-conducting types, they can not be directly integrated into silicon-based micro-fabrication” adds Dr. Aravind Vijayaraghavan. “Due to the difficulties in handling and manipulating these nano-scale objects at the individual level, various attempts to assemble them into functional devices have met with limited success. In the ideal case, it should be possible to position an individual nanotube at a predefined location and orientation, forming robust, low-resistance, ohmic contacts to two metallic leads. Furthermore, it should be possible to do this at a scalable integration density with each nanotube forming an individually addressable device.”
Krupke and Vijayaraghavan are scientists at the Institute of Nanotechnology (INT) at the Research Center Karlsruhe in Germany. Together with colleagues from the INT and the University of Karlsruhe they authored a recent paper in Nano Letters, titled “Ultra-Large-Scale Directed Assembly of Single-Walled Carbon Nanotube Devices”. In it, they report a novel aspect of dielectrophoretic deposition of CNTs, where the dielectrophoretic force field changes upon nanotube deposition and thereby self limits the directed assembly to a single nanotube or nanotube bundle at predefined locations.
In 2003, the group demonstrated that it is possible to deposit CNT bundles from an aqueous solution using a process called dielectrophoresis which uses inhomogeneous alternating electric fields to move and assemble nano-scale objects.
“Since then, we have made tremendous advances in understanding the dynamics of a carbon nanotube moving in such an electric field” says Vijayaraghavan. “The required inhomogeneous electric fields are generated by two opposing needle-shaped electrodes with a microscopic gap between their tips. We have discovered the mechanism that allows for a self-limiting deposition of CNTs to one per electrode pair. This happens because the first CNT that is deposited in the gap changes the electric field distribution around it incisively, leading to a repulsion of subsequent CNTs that attempt to enter the region of the gap.”
The researchers in Karlsruhe have also developed and optimized the use of capacitively coupled electrodes, which enables them to reduce their dimensions and increase the density of electrode pairs that can be incorporated on a chip.
“Together, this allows us to fabricate separately addressable, individual SWCNT devices at an integration density comparable to ultra-large scale integration” says Krupke. “This is three to four orders of magnitude greater than what has been possible so far with any other technique.”
This technique is very versatile. It is compatible with SWCNTs from any source, which are suitably dispersed in an aqueous surfactant solution. SWCNTs separated based on their length, diameter or even chirality can be readily assembled into large-scale functional arrays using this technique. The process is fully compatible with post-processing techniques and current microelectronics fabrication technologies, requires no high-temperature steps or chemical modification of the substrate or the CNT and is a one-step process that can be performed under ambient conditions.
This achievement takes CNT electronic devices a big step closer to integrating with microelectronics and expanding their scope for commercial viability. On a laboratory scale, it now allows for the fabrication of a large number of devices with identical CNT source and deposition conditions, to perform truly statistical measurements of CNT properties like electronic transport or Raman mapping.
By Michael Berger, Copyright 2007 Nanowerk LLC
Watch amazing footage of how nanotubes form
11th May 2007
Here’s an interesting piece which shows on video how carbon nanotubes form. It looks to be a follow up of some work done in March at Cambridge.
Watch amazing footage of how nanotubes form

Environmental transmission electron microscopy image sequence of carbon nanofibre growth. Drawings (lower row) indicate schematically the Ni catalyst deformation and C-Ni interface. Credit: University of Cambridge
A team of scientists led by the Department’s Dr Stephan Hofmann have successfully produced live video footage that shows how carbon nanotubes, more than 10,000 times smaller in diameter than a human hair, form.
The video sequences can be viewed by clicking the links below. They show nanofibres and nanotubes nucleating around miniscule particles of nickel and are already offering greater insight into how these microscopic structures self-assemble.
These two videos show how the nickel reacts a process called catalytic chemical vapour deposition (CVD). This is one of several methods of producing nanotubes, and involves the application of a gas containing carbon (in this case acetylene) to minute crystalline droplets referred to as “catalyst islands” (the nickel).
In conditions appropriate to creating nano-fibres, the catalyst was squeezed upwards gradually as carbon formed around it. When the application of gas was reduced to create single-walled nanotubes, the carbon instead lifted off the catalyst to form a tubular structure.
Nanotube movie 1
Nanotube movie 2
In particular, the team discovered that the carbon network is guided into tubular shape by a drastic restructuring of the nickel – the catalyst in the process. They were also able to track and time the deposition of the carbon around the nickel.
Carbon nanotubes are new building blocks enabling engineers to improve and further miniaturise everyday electronic devices like computers or mobile phones. At the moment scientists can grow nanotubes but cannot accurately control their structure.
Being able to do so is vital as it is the very structure of a nanotube that dictates its properties. The nano-scale video observations mean that scientists will be able to better understand the nucleation of nanotubes and are therefore an important step on the route towards application.
The two sequences show action taking place in real time on an astonishingly small scale. The difference in size between a single-walled nanotube and a human hair is close to the difference between the same human hair and the Eiffel Tower. The microscopic scale involved has, in the past, made it difficult to understand the growth process.
The team used X-rays produced at a synchrotron (a type of particle accelerator) and a modified high-resolution transmission electron microscope to observe and film the catalytic chemical vapour deposition process.
As the gas is applied carbon sticks to the catalyst islands forming layers of graphite. In conditions appropriate to creating nanofibres, the nickel particle was pushed upwards in a series of peristaltic movements as the carbon continued to deposit on its sides. At several points the nickel formed a cap which almost “popped” out of the forming tube, leaving a layer of graphite behind it. This process is called “bambooing”, because the resultant carbon nanofibre is a cylinder containing several cavities, each one separated by one of these graphite layers, similar in form to bamboo. Throughout the whole process, the nickel remained crystalline rather than liquid.
The team then looked at conditions more appropriate to producing single-walled carbon nanotubes, which involved less acetylene. The catalyst is not squeezed upwards. Instead, a cap of carbon formed on the top of the nickel, and gradually extended from it to form a tubular structure. The catalyst island was squeezed and reshaped by this process and was moulded by the carbon forming around it rather than retaining its original form.
Dr Stephan Hofmann, who led the research, said: “In order to reach the full application potential for nanotubes, we need to be able to accurately control their growth first. As a manifestation of the impressive progress of nanometrology, we are actually now able to watch molecular objects grow. This new video footage shows that the catalyst itself remains crystalline but is constantly changing its shape as the carbon network is formed around it.
“We cannot yet solve the problem of not being able to self-assemble carbon nanotubes with well-defined characteristics, but we have discovered that if we are to do so, we need to be mindful not just of the carbon dynamics but the changing shape of the catalyst as well.”
Source: University of Cambridge
This news is brought to you by PhysOrg.com




